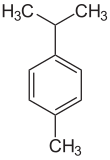
P-Cymene
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Ad Genius Lee Tae-baekPoster promosi unutk Ad Genius Lee Tae-baekGenreDrama tempat kerja RomansaDitulis olehSeol Joon-seok Lee Jae-ha Lee Yoon-jongSutradaraPark Ki-ho Lee So-yeonPemeranJin GooPark Ha-sunJo Hyun-jaeHan Chae-young LPenata musikOh Joon-seong Lee Im-wooNegara asalKorea SelatanBahasa asliKoreaJmlh. episode16ProduksiProduser eksekutifLee Jae-young Yoo Geon-shikProduserKim Jin-wooLokasi produksiKoreaSinematografiUhm Joon-seong Lee Young-seobPenyuntingHan Ok-geumDurasi60 menit...

العلاقات السعودية الكاميرونية السعودية الكاميرون السعودية الكاميرون تعديل مصدري - تعديل العلاقات السعودية الكاميرونية هي العلاقات الثنائية التي تجمع بين السعودية والكاميرون.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للد�...

Bagian dari seri artikel mengenaiPerang Sejarah Prasejarah Kuno Abad pertengahan Modern awal Industri Modern Perang generasi keempat Ruang pertempuran Udara Dunia maya Informasi Antariksa Darat Wilayah dingin Gurun Hutan Gunung Perkotaan Bawah tanah Laut Amfibi Biru Cokelat Hijau Permukaan Bawah air Siber Informasi Senjata Lapis baja Artileri Biologi Kavaleri Kimia Konvensional Dunia maya Elektronik Infanteri Nuklir Psikologi Nonkonvensional Taktik Udara Pertempuran Kavaleri Serbuan Serangan ...

Bay in Limestone Coast, South AustraliaGuichen BayThe Robe ObeliskGuichen BayLocation in South AustraliaLocationLimestone Coast, South AustraliaCoordinates37°7′14″S 139°45′58″E / 37.12056°S 139.76611°E / -37.12056; 139.76611[1]TypeBayBasin countriesAustraliaMax. length6 km (3.7 mi)[2]Max. width3.4 km (2.1 mi)[2]Max. depth11.3 m (37 ft)[2]SettlementsRobe Guichen Bay, (locally /ˈɡiːʃən/ ...

Medical service network University of Vermont Health Network redirects here Hospital in Vermont, U.S.University of Vermont Medical CenterUVM Medical Center Main CampusGeographyLocation111 Colchester Avenue, Burlington, Vermont, U.S.Coordinates44°28′47″N 73°11′39″W / 44.479794°N 73.194119°W / 44.479794; -73.194119OrganisationTypeTeachingAffiliated universityUniversity of VermontServicesEmergency departmentIBeds620HelipadYes (FAA LID: 67VT)HistoryOpened1879&#...

Mona AmbegaonkarMona Ambegaonkar dalam film Evening ShadowsLahir5 Maret 1970 (umur 54)Mumbai, Maharashtra, IndiaKebangsaanIndiaPekerjaanaktrisAnak1 Mona Ambegaonkar (kelahiran 5 Maret 1970) adalah seorang aktris film dan televisi India.[1] Ia telah tampil dalam lebih dari 15 drama, 18 film fitur, 38 proyek TV, 37 kampanye iklan. Ia memainkan sebuah peran kecil dalam film Hazaar Chaurasi Ki Maa dan drama medis Dhadkan sebagai Dr. Chitra.[1] Filmografi Film Tahun Judul Per...

Piala Liga Irlandia UtaraMulai digelar1986Wilayah Irlandia UtaraJumlah tim42Juara bertahanCliftonvilleTim tersuksesLinfield(9 gelar) 2013–14 Irish League Cup Piala Liga Irlandia Utara (Inggris: Irish League Cup), atau IRN-BRU League Cup (Inggris: IRN-BRU League Cup) untuk alasan sponsor,[1] adalah kejuaraan sepak bola yang diselenggarakan di Irlandia Utara yang diikuti oleh peserta Liga Irlandia Utara. Kejuaraan ini dimulai sejak tahun 1986. Daftar juara Musim Juara Sko...

Historical South African Nazist movement/paramilitary For other uses, see Greyshirt (disambiguation). Greyshirts or Gryshemde is the common short-form name given to the South African Gentile National Socialist Movement, a South African Nazi movement that existed during the 1930s and 1940s. Initially referring only to a paramilitary group, it soon became shorthand for the movement as a whole. The NSDAP/AO arrived in South Africa in 1932 and as a result a number of groups sympathetic to Nazism ...

1978 single by AmbrosiaHow Much I FeelSingle by Ambrosiafrom the album Life Beyond L.A. B-sideReady for CamarilloReleasedAugust 1978Recorded1978GenreSoft rock[1][2]Length4:46LabelWarner Bros. 8640Songwriter(s)David PackProducer(s)Freddie PiroAmbrosiaAmbrosia singles chronology Magical Mystery Tour (1977) How Much I Feel (1978) Life Beyond L.A. (1978) How Much I Feel is a 1978 song by American rock band Ambrosia. The song, written by the band's guitarist/vocalist David Pack, wa...

本條目存在以下問題,請協助改善本條目或在討論頁針對議題發表看法。 此條目可能包含原创研究。 (2018年3月29日)请协助補充参考资料、添加相关内联标签和删除原创研究内容以改善这篇条目。详细情况请参见讨论页。 此條目需要补充更多来源。 (2010年2月4日)请协助補充多方面可靠来源以改善这篇条目,无法查证的内容可能會因為异议提出而被移除。致使用者:请搜索一�...

密西西比州 哥伦布城市綽號:Possum Town哥伦布位于密西西比州的位置坐标:33°30′06″N 88°24′54″W / 33.501666666667°N 88.415°W / 33.501666666667; -88.415国家 美國州密西西比州县朗兹县始建于1821年政府 • 市长罗伯特·史密斯 (民主党)面积 • 总计22.3 平方英里(57.8 平方公里) • 陸地21.4 平方英里(55.5 平方公里) • ...

Comics character Brother Power the GeekPublication informationPublisherDC Comics/VertigoFirst appearanceBrother Power the Geek #1 (October 1968)Created byJoe SimonIn-story informationAlter egoBrother PowerTeam affiliationsLove Syndicate of DreamworldAbilitiesPuppet Elemental: (Pre-Vertigo): superhuman strength, electricity absorption, superhuman leaping ability, durability, somewhat higher intelligence, albeit limited in formal education (Vertigo): ability to reside in any artificial figure r...

تصوير ثنائي الأبعاد من مسافة هامنج، يعد مقياسا حاسما في نظرية الترميز. نظريه الترميز أو نظرية التكويد (بالإنجليزية: Coding theory) نظرية الترميز هي دراسة خصائص الرموز ونقاط القوه الخاصة بها من اجل تطبيقات محددة. وتستخدم الرموز لضغط البيانات، التشفير، تصحيح الخطأ، والشبكات. الر...

Not to be confused with Mapperton. Human settlement in EnglandMapertonChurch of St Peter and St Paul. MapertonMapertonLocation within SomersetPopulation140 (2011)[1]OS grid referenceST675265DistrictSouth SomersetShire countySomersetRegionSouth WestCountryEnglandSovereign stateUnited KingdomPost townWINCANTONPostcode districtBA9Dialling code01963PoliceAvon and SomersetFireDevon and SomersetAmbulanceSouth Western UK ParliamentSomerton and Frome Li...

Species of mammal Dice's cottontail Conservation status Vulnerable (IUCN 3.1)[1] Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Class: Mammalia Order: Lagomorpha Family: Leporidae Genus: Sylvilagus Species: S. dicei Binomial name Sylvilagus diceiHarris, 1932 Dice's cottontail range Dice's cottontail (Sylvilagus dicei) is a species of cottontail rabbit in the family Leporidae. It is found in Costa Rica and Panama, in páramo and cloud forest h...

Yahya Muhaimin Menteri Pendidikan Nasional Indonesia ke-23Masa jabatan29 Oktober 1999 – 23 Juli 2001PresidenAbdurrahman WahidPendahuluJuwono SoedarsonoPenggantiAbdul Malik Fadjar Informasi pribadiLahir(1943-05-17)17 Mei 1943Bumiayu, BrebesMeninggal9 Februari 2022(2022-02-09) (umur 78)[1]Purwokerto, BanyumasPartai politikIndependenAnak1Alma materUniversitas Gadjah MadaMassachusetts Institute of TechnologyPekerjaanPengajarDosenSunting kotak info • L • B...

Defunct art museum in New York City Met BreuerMET Breuer Building (2019)EstablishedMarch 18, 2016 (2016-03-18)DissolvedMarch 13, 2020 (temporary closure)June 23, 2020 (permanent closure)Location945 Madison Avenue, New York, NY 10021TypeArt museumPublic transit access Subway: at 77th Street Bus: M1, M2, M3, M4, M79 SBSWebsitemetmuseum.org/visit/met-breuer The Met Breuer (/ˈbrɔɪ.ər/ BROY-ər)[1] was a museum of modern and contemporary art at Madison Avenue and...

Cankiri province Çankırı iliProvince of TurkeyLocation of Cankiri Province in TurkeyCountryTurkeyRegionCentral AnatoliaLuas • Total7.388 km2 (2,853 sq mi)Populasi (2010-12-31)[1] • Total179.067 • Kepadatan0,024/km2 (0,063/sq mi)Kode area telepon0376Pelat kendaraan18Situs webcankiri.gov.tr Çankırı (Turki: Çankırı ili) adalah sebuah provinsi Turki. lbsDaftar provinsi Turki Adana · Adıyaman · ...

NS22 Stasiun MRT Orchard乌节地铁站ஆர்ச்சர்ட்Angkutan cepatPeron Stasiun NS22 MRT OrchardLokasi437 Orchard RoadSingapore 238878Koordinat1°18′15.53″N 103°49′54.98″E / 1.3043139°N 103.8319389°E / 1.3043139; 103.8319389Jalur Jalur Utara Selatan Jumlah peronPulauJumlah jalur2LayananBus, TaksiKonstruksiJenis strukturBawah tanahTinggi peron2Akses difabelYesInformasi lainKode stasiunNS22SejarahDibuka12 Desember 1987Ope...

Port in United StatesPort of WilmingtonThe Port of Wilmington as seen from Interstate 495 on July 9, 2011.Click on the map for a fullscreen viewLocationCountryUnited StatesLocationWilmington, DelawareCoordinates39°43′06″N 75°31′25″W / 39.71833°N 75.52361°W / 39.71833; -75.52361 (Port of Wilmington (Delaware))UN/LOCODEUSILG[1]DetailsOpened1923Operated byPort WilmingtonOwned byDiamond State Port CorporationLand area308 acres (1.25 km2)No....