Mitsunobu reaction
| |||||||||||||||||||||||||||
Read other articles:

The Dilemmas of Lenin: Terrorism, War, Empire, Love, Revolution PengarangTariq AliSubjekNonfiksiPenerbitVerso BooksTanggal terbit2017Jenis mediaCetakHalaman384 halaman The Dilemmas of Lenin: Terrorism, War, Empire, Love, Revolution adalah sebuah buku tahun 2017 yang ditulis oleh aktivis dan Trotskyis Tariq Ali, yang berfokus pada kehidupan revolusioner Bolshevik Rusia Vladimir Lenin.[1][2] Referensi ^ Ali, Tariq (2017). The Dilemmas of Lenin: Terrorism, War, Empire, ...

Imperial Guard近衛師団Panji dari Tentara Kekaisaran JepangAktif1891–1945Negara Empire of JapanAliansi Kaisar JepangTipe unitInfanteriInfanteri bermotor (1940-)Jumlah personel3 (divisi)MarkasTokyoPertempuranPerang Tiongkok-JepangPerang Rusia-JepangInsiden 26 FebruariPeristiwa KyūjōPertempuran MalayaPertempuran SingapuraDibubarkan1945 Pengawal Kekaisaran (近衛師団code: ja is deprecated , Konoe Shidan) adalah salah satu divisi dari Angkatan Darat Kekaisaran Jepang. Berbeda denga...

Sebuah laman dari bagian Bhawishyottara dari Bhawisyapurana (Sanskerta, Dewanagari) Bagian dari seriSastra Hindu Weda Regweda Samaweda Yajurweda Atharwaweda Pembagian Weda Samhita Brahmana Aranyaka Upanisad Upanisad Aitareya Brihadaranyaka Isa Taittiriya Chandogya Kena Mundaka Mandukya Prasna Swetaswatara Wedangga Siksha Chanda Wyakarana Nirukta Jyotisha Kalpa Itihasa Mahabharata Ramayana Susastra lainnya Smerti Purana Bhagawadgita Sutra Pancaratra Tantra Kumarawyasabharata Stotra Hanumancali...

State park in California, United States Gray Whale Cove State BeachShow map of CaliforniaShow map of the United StatesLocationSan Mateo County, California, United StatesNearest cityMontara, CaliforniaCoordinates37°33′56″N 122°30′52″W / 37.56556°N 122.51444°W / 37.56556; -122.51444Area3.1 acres (1.3 ha)Established1966Governing bodyCalifornia Department of Parks and Recreation Gray Whale Cove State Beach is a California State Park between Pacif...

For related races, see 1962 United States gubernatorial elections. 1962 Arkansas gubernatorial election ← 1960 November 6, 1962 1964 → Nominee Orval Faubus Willis Ricketts Party Democratic Republican Popular vote 225,743 82,349 Percentage 73.27% 26.73% County results Faubus: 50–60% 60–70% 70–80% 80–90% ...

Henry Brevard DavidsonBrig. Gen. Henry B. DavidsonBorn(1831-01-28)January 28, 1831Shelbyville, TennesseeDiedMarch 4, 1899(1899-03-04) (aged 68)Livermore, CaliforniaPlace of burialMountain View CemeteryAllegiance United States of America Confederate States of AmericaService/branch United States Army Confederate States ArmyYears of service1846–1847, 1853–1861 (USA)1861–1865 (CSA)Rank Captain (USA) Brigadier General (CSA)Commands heldCavalry brigade in ...

46-я церемония вручения наград премии «Золотой глобус» 28 января 1989 года Лучший фильм (драма): «Человек дождя» Лучший фильм (комедия или мюзикл): «Деловая девушка» Лучший драматический сериал: «Тридцать-с-чем-то» Лучший сериал (комедия или мюзикл): «Чудесные годы» Лучший ми�...

1998 single by Jay-Z Can I Get A... (Soundtrack Version)Sticker from shrink wrap of the U.S. 12-inch vinyl singleSingle by Jay-Z featuring Jah and Amilfrom the album Def Jam's Rush Hour Soundtrack and Vol. 2... Hard Knock Life ReleasedAugust 22, 1998 (1998-08-22)Recorded1998GenreHip hopelectro-disco[1]Length5:11LabelDef JamPolyGramSongwriter(s)Shawn CarterJeffrey AtkinsIrving LorenzoRobin MaysProducer(s)Irv GottiLil' RobJay-Z singles chronology Money Ain't a Thang(1...

Josip BozanićKardinal, Uskup Agung ZagrebKeuskupan agungKeuskupan Agung ZagrebProvinsi gerejawiZagrebTakhtaZagrebPenunjukan5 Juli 1997PendahuluFranjo KuharićJabatan lainKardinal-Imam S. Girolamo dei Croati (degli Schiavoni)ImamatTahbisan imam29 Juni 1975oleh Karmelo ZazinovićTahbisan uskup25 Juni 1989oleh Franjo KuharićPelantikan kardinal21 Oktober 2003PeringkatKardinal-ImamInformasi pribadiNama lahirJosip BozanićLahir20 Maret 1949 (umur 75)Rijeka, KroasiaKewarganegaraanKr...
犹太人יהודים(Yehudim)雅各耶稣大卫王爱因斯坦马克思迈蒙尼德弗拉维奥·约瑟夫斯弗洛伊德斯宾诺莎本-古里安西奥多·赫茨尔娜塔莉·波特曼弗里茨·哈伯冯诺依曼門德爾頌谢尔盖·布林罗莎·卢森堡莉泽·迈特纳乔姆斯基维特根斯坦大卫·李嘉图尼尔斯·玻尔赛尔曼·瓦克斯曼卡夫卡史翠珊泽连斯基罗莎琳德·富兰克林古斯塔夫·马勒普鲁斯特卡米耶·毕沙罗涂尔干摩西...

Сон Сенкхмер. សុន សេន Министр обороны Камбоджи 15 января 1976 — 7 января 1979 Глава правительства Пенн НутПол Пот Президент Кхиеу Сампхан Преемник Пен Сован Рождение 12 июня 1930(1930-06-12)Чавинь, Южный Вьетнам Смерть 10 июня 1997(1997-06-10)[1] (66 лет) или 15 июня 1997(1997-06-15) (67 лет)А�...

World War II merchant ship of the United Kingdom History Name Belgia (1930-41) Empire Bell (1941-42) Owner Förnyade Ångfartygs Aktiebolag Götha (1930-41) Ministry of War Transport (1941-42) Operator F Sternhagen - Gotha Line (1930-41) James Westroll Ltd, Sunderland (1941-42) Port of registry Gothenborg (1930-41) South Shields (1940-41) BuilderÖresundsvarvet, Landskrona Yard number28 Launched11 January 1930 Completed7 May 1930 Out of service25 September 1942 Identification Swedish Official...

Island and neighbourhood of Helsinki, Finland This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Santahamina – news · newspapers · books · scholar · JSTOR (January 2012) (Learn how and when to remove this message) Location of Santahamina within Helsinki Hevossalmi bridge and road to Santahamina Santahamina (Swedish: Sandhamn) is...

Seventh year of the seven-year agricultural cycle mandated by the Torah for the Land of Israel You can help expand this article with text translated from the corresponding article in Hebrew. Click [show] for important translation instructions. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machine-translated text into ...

此条目序言章节没有充分总结全文内容要点。 (2019年3月21日)请考虑扩充序言,清晰概述条目所有重點。请在条目的讨论页讨论此问题。 哈萨克斯坦總統哈薩克總統旗現任Қасым-Жомарт Кемелұлы Тоқаев卡瑟姆若马尔特·托卡耶夫自2019年3月20日在任任期7年首任努尔苏丹·纳扎尔巴耶夫设立1990年4月24日(哈薩克蘇維埃社會主義共和國總統) 哈萨克斯坦 哈萨克斯坦政府...
2013 Milwaukee IndyFestRace details9th round of the 2013 IndyCar Series seasonDateJune 15, 2013Official nameMilwaukee IndyFestLocationMilwaukee MileCoursePermanent racing facility1.015 mi / 1.633 kmDistance225 laps228.38 mi / 367.542 kmWeatherTemperatures reaching up to 73.4 °F (23.0 °C); wind speeds approaching 8 miles per hour (13 km/h)[1]Pole positionDriverMarco Andretti (Andretti Autosport)PodiumFirstRyan Hunter-Reay (Andretti Autosport)SecondHéli...

هذه المقالة عن قناة أمريكية ناطقة باللغة العربية. لقناة ليبية ظهرت في ثورة 17 فبراير، طالع ليبيا الحرة. الحرة معلومات عامة النوع شبكة تلفزيونية فضائية المالك ميدل إيست برودكاستنغ نيتوورك (الحكومة الأمريكية) تاريخ التأسيس 2004 البلد الولايات المتحدة اللغة ال...

حمزة نمرة حمزة نمرة في إحدى حفلاته معلومات شخصية اسم الولادة حمزة حسام مصطفى الميلاد 15 نوفمبر 1980 (44 سنة) الدرعية الإقامة لندن مواطنة مصر عدد الأولاد 3 الحياة الفنية النوع ترتيل، وموسيقى شعبية، وريذم أند بلوز الآلات الموسيقية قيثارة،...
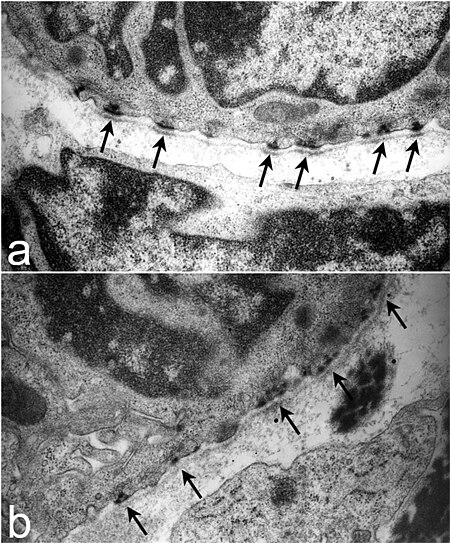
HemidesmosomeUltrastructure of tracheal hemidesmosomes in mice. In a normal mouse (a) there are well-defined, organized hemidesmosomes with darkened areas in the lamina densa abutting the hemidesmosome (arrows). In contrast, hemidesmosomes in Lamc2 -/- tracheas (b) are less organized, the intracellular component is more diffuse, and the lamina densa directly below the hemidesmosomal areas lacks the electron density seen in the littermate control (arrows). From Nguyen et al., 2006.[1]D...

У этого термина существуют и другие значения, см. Ло. ДепартаментЛофр. Lotокс. Òlt, Òut Флаг Герб 44°35′ с. ш. 1°35′ в. д.HGЯO Страна Франция Входит в Окситания Включает 3 округов, 31 кантонов и 340 коммун Адм. центр Каор Председатель генерального совета Серж Ригаль Ист�...




