Hemidesmosome
| |||||||||||||||||||
Read other articles:

Rosita van Gijlswijk Lia Rosita van Gijlswijk (lahir 11 Februari 1974) adalah seorang politikus Belanda dari Partai Sosialis. Ia menjadi anggota parlemen dari 2006 sampai 2008; ia digantikan oleh Farshad Bashir. Dari 1999 sampai 2006, ia menjadi anggota dewan munisipal Groningen; sejak 2008, ia kembali menjadi konselor munisipalitas tersebut. Pada 2007, ia menjadi bendahara Partai Sosialis.[1] Referensi (dalam bahasa Belanda) Parlement.com biography ^ Rosita van Gijlswijk wo...

Untuk informasi tentang Wikidata di Wikipedia, lihat Wikipedia:Wikidata.artikel ini perlu dirapikan agar memenuhi standar Wikipedia. Tidak ada alasan yang diberikan. Silakan kembangkan ini semampu Anda. Merapikan artikel dapat dilakukan dengan wikifikasi atau membagi artikel ke paragraf-paragraf. Jika sudah dirapikan, silakan hapus templat ini. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) WikidataHalaman utama pada April 2021URLwikidata.org Eponimwiki dan Data Nama sing...

Gabriel Duvall Hakim Mahkamah Agung Amerika SerikatMasa jabatan23 November 1811 – 14 Januari 1835 Informasi pribadiKebangsaanAmerika SerikatProfesiHakimSunting kotak info • L • B Gabriel Duvall adalah hakim Mahkamah Agung Amerika Serikat. Ia mulai menjabat sebagai hakim pada mahkamah tersebut pada tanggal 23 November 1811. Masa baktinya sebagai hakim berakhir pada tanggal 14 Januari 1835.[1] Referensi ^ Justices 1789 to Present. Washington, D.C.: Mahkamah Agun...

American politician This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Grant Sawyer – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this template message) Grant SawyerChair of the National Governors AssociationIn officeJune 6, 1964 – July 25, 1965Preceded b...

بارغا تقسيم إداري البلد اليونان [1] خصائص جغرافية إحداثيات 39°17′00″N 20°24′00″E / 39.283333333333°N 20.4°E / 39.283333333333; 20.4 المساحة 275 كيلومتر مربع الارتفاع 32 متر السكان التعداد السكاني 2088 (إحصاء السكان) (2011)2022 (resident population of Greece) (2001)1767 (resident population of Greece) (1991)2216 ...

Kedutaan Besar Republik Indonesia di AstanaKoordinat51°08′03″N 71°25′31″E / 51.134176°N 71.425226°E / 51.134176; 71.425226Lokasi Astana, KazakhstanAlamat22 Saraishyk StreetAstana, 010000, KazakhstanDuta BesarFadjroel RachmanYurisdiksi Kazakhstan TajikistanSitus webkemlu.go.id/astana/id Kedutaan Besar Republik Indonesia di Astana (KBRI Astana) adalah misi diplomatik Republik Indonesia untuk Kazakhstan dan merangkap sebagai perwakilan Indonesia untu...

Voce principale: Aurora Pro Patria 1919. Pro Patria Gallaratese G.B.Stagione 2000-2001Sport calcio Squadra Pro Patria Allenatore Gianfranco Motta Presidente Alberto Armiraglio Serie C23º posto (playoff persi) Coppa ItaliaPrimo turno Maggiori presenzeCampionato: Toniolo (32) Miglior marcatoreCampionato: Porfido (11) StadioStadio Carlo Speroni 1999-2000 2001-2002 Si invita a seguire il modello di voce Questa voce raccoglie le informazioni riguardanti la Pro Patria Gallaratese G.B. nelle ...
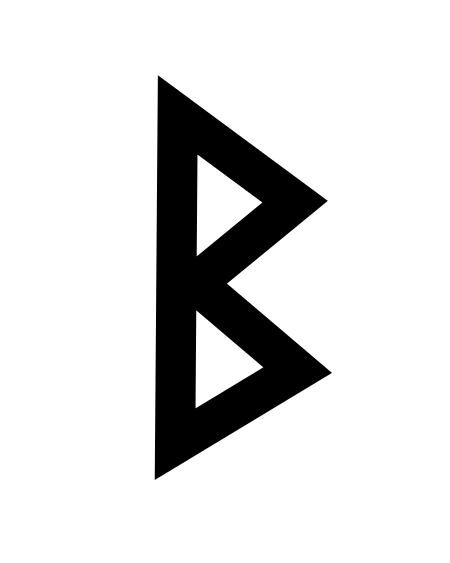
此條目介紹的是拉丁字母中的第2个字母。关于其他用法,请见「B (消歧义)」。 提示:此条目页的主题不是希腊字母Β、西里尔字母В、Б、Ъ、Ь或德语字母ẞ、ß。 BB b(见下)用法書寫系統拉丁字母英文字母ISO基本拉丁字母(英语:ISO basic Latin alphabet)类型全音素文字相关所属語言拉丁语读音方法 [b][p][ɓ](适应变体)Unicode编码U+0042, U+0062字母顺位2数值 2歷史發...

Alessandro BarnabóPrefek Kongregasi bagi Penyebaran ImanGerejaGereja Katolik RomaPenunjukan20 Juni 1856Masa jabatan berakhir24 Februari 1874PendahuluGiacomo Filippo FransoniPenerusAlessandro FranchiJabatan lainKardinal-Imam Santa Susanna (1856–74)ImamatTahbisan imamMaret 1833Pelantikan kardinal16 Juni 1856oleh Paus Pius IXPeringkatKardinal-ImamInformasi pribadiNama lahirAlessandro BarnabòLahir2 Maret 1801Foligno, Negara GerejaWafat24 Februari 1874(1874-02-24) (umur 72)Roma, Negara Ge...

Kolkata Municipal Corporation in West Bengal, IndiaWard No. 124Kolkata Municipal CorporationInteractive Map Outlining Ward No. 124Ward No. 124Location in KolkataCoordinates: 22°28′07″N 88°19′00″E / 22.468675°N 88.316694°E / 22.468675; 88.316694Country IndiaStateWest BengalCityKolkataNeighbourhoodsBarisha, ThakurpukurReservationOpenParliamentary constituencyKolkata DakshinAssembly constituencyBehala PurbaBorough16Population (2011) • To...

この記事は検証可能な参考文献や出典が全く示されていないか、不十分です。出典を追加して記事の信頼性向上にご協力ください。(このテンプレートの使い方)出典検索?: コルク – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサーチ · TWL(2017年4月) コルクを打ち抜いて作った瓶の栓 コルク(木栓、�...

BellevilleQuartier administratifQuartier de BellevilleParc de BellevilleKoordinat: 48°52′26″N 2°23′07″E / 48.87389°N 2.38528°E / 48.87389; 2.38528Koordinat: 48°52′26″N 2°23′07″E / 48.87389°N 2.38528°E / 48.87389; 2.38528Negara PrancisRegionÎle-de-FranceKomuneParisArondisemenke-20Luas • Total0,807 km2 (0,312 sq mi)Populasi (2016)[1] • Total35,605 • Kepad...

此条目序言章节没有充分总结全文内容要点。 (2019年3月21日)请考虑扩充序言,清晰概述条目所有重點。请在条目的讨论页讨论此问题。 哈萨克斯坦總統哈薩克總統旗現任Қасым-Жомарт Кемелұлы Тоқаев卡瑟姆若马尔特·托卡耶夫自2019年3月20日在任任期7年首任努尔苏丹·纳扎尔巴耶夫设立1990年4月24日(哈薩克蘇維埃社會主義共和國總統) 哈萨克斯坦 哈萨克斯坦政府...

Voce principale: Akademia Sant'Anna. Akademia Sant'AnnaStagione 2022-2023Sport pallavolo Squadra Sant'Anna Allenatore Marco Breviglieri, poi Fabio Bonafede All. in seconda Flavio Ferrara Presidente Fabrizio Costantino Serie A29ª (girone B) Pool salvezza4ª Maggiori presenzeCampionato: Ebatombo, Martilotti, Muzi (31)Totale: Ebatombo, Martilotti, Muzi (31) Miglior marcatoreCampionato: Ebatombo (422)Totale: Ebatombo (422) 2021-22 2023-24 Questa voce raccoglie le informazioni riguardanti l...

Boeing Model 247 adalah pesawat awal sayap rendah (low wing) Amerika Serikat, dianggap sebagai pesawat yang pertama untuk sepenuhnya menggabungkan[1][2] kemajuan seperti semua logam (aluminium anodized) konstruksi semi-monocoque, sayap kantilever dan landing gear tarik. Fitur canggih lainnya termasuk kontrol permukaan memangkas tab, autopilot dan deicing sepatu untuk sayap dan tailplane.[3] Referensi ^ Model 247 Commercial Transport. boeing.com, 2009. Retrieved: June ...

Radio station in Columbia, South CarolinaWNOKColumbia, South CarolinaBroadcast areaColumbia metropolitan areaMidlands of South CarolinaFrequency104.7 MHz (HD Radio)Branding104.7 WNOKProgrammingFormatTop 40 (CHR)AffiliationsCompass Media NetworksPremiere NetworksOwnershipOwneriHeartMedia, Inc.(iHM Licenses, LLC)Sister stationsWCOS, WCOS-FM, WLTY, WVOC, WXBTHistoryFirst air dateJuly 15, 1959Technical information[1]Licensing authorityFCCFacility ID19472ClassC1ERP90,000 wattsHAAT315 meter...

Kolam vernal pada akhir musim dingin di Ewing Township, Mercer County, New Jersey, Amerika Serikat. Kolam vernal adalah cekungan dangkal berisi air di permukaan tanah yang terbentuk secara alami pada musim tertentu. Di Maine dan Massachusetts, kolam vernal terisi oleh air hujan. Banyak kolam vernal di timur laut Amerika Serikat tertutup es pada musim dingin. Kolam vernal terdapat di California, yang terbesar di San Diego. Di luar Amerika Serikat, kolam vernal banyak terdapat di Spanyol. Secar...

Questa voce sull'argomento calciatori polacchi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Wojciech RudyRudy (a destra), capitano della Polonia, saluta Dino Zoff prima dell'amichevole con l'Italia del 1980.Nazionalità Polonia Altezza175 cm Peso73 kg Calcio RuoloDifensore Termine carriera1985 CarrieraSquadre di club1 1970-1983 Zagłębie Sosnowiec272 (13)1983-1984 KuPS32 (7)1984-1985...

Нефтеперерабатывающий завод в Баку, 1912 Операция Pike (англ. pike — щука или острие копья) — кодовое название англо-французского плана стратегических бомбардировок бакинских нефтепромыслов в начальный период Второй мировой войны. Реализован не был. Несмотря на форм�...

1969–1976 opposition government and state in South Vietnam For the Republic of Vietnam which is commonly known as South Vietnam, see South Vietnam. For the entity known from 1948 to 1949 as the Provisional Government of South Vietnam, see French Cochinchina. This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Provisional Revolutionary Government...
