Dalbavancin
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

The Right HonourableJoseph Philippe Pierre Yves Elliott TrudeauPC CC CH QC LLD (Mont) MA MSRCTrudeau pada resepsi oleh Ratu Juliana dari Belanda, pada Februari 1975 Perdana Menteri Kanada Ke-15Masa jabatan20 April 1968 – 4 Juni 1979Penguasa monarkiElizabeth II PendahuluLester B. PearsonPenggantiJoe ClarkMasa jabatan3 Maret 1980 – 30 Juni 1984 PendahuluJoe ClarkPenggantiJohn TurnerKetua Kelompok Oposisi ke-18Masa jabatan4 Juni 1979 – 2 Maret 1980 PendahuluJ...

متحف التاريخ والحضارات إحداثيات 34°00′49″N 6°49′53″W / 34.013611°N 6.831389°W / 34.013611; -6.831389 معلومات عامة الموقع الرباط العنوان Archaeological Museum 23 rue Brihi القرية أو المدينة الرباط الدولة المغرب سنة التأسيس 1932 تاريخ الافتتاح الرسمي 1986 المساحة ؟؟؟ متر مربع معلومات أخرى ال�...

Kabinet DaruratKabinet Pemerintahan IndonesiaRumah ketua PDRI Sjafroedin Prawiranegara di Bidar Alam yang dipergunakan juga untuk kantor pemerintahanDibentuk19 Desember 1948Diselesaikan13 Juli 1949Struktur pemerintahanKepala negaraSukarnoKepala pemerintahanSyafruddin PrawiranegaraSejarahPendahuluKabinet Hatta IPenggantiKabinet Hatta II Artikel ini bagian dariseri tentangSoekarno Presiden pertama Indonesia Prakemerdekaan PNI Partindo PETA BPUPK Pancasila PPKI Revolusi Nasional Indonesia Prokla...

Untuk politisi, lihat Jerry Lawalata. Jerry O'ConnellJerry O'Connell, 2008LahirJames Raymond Lawalata17 Februari 1974 (umur 50)Kota New York, Amerika SerikatPekerjaanAktorTahun aktif1986–sekarangSuami/istriRebecca Romijn (m. 2007–sekarang; 2 anak) Jeremiah Jerry O'Connell (lahir 17 Februari 1974) adalah aktor asal Amerika Serikat yang dikenal karena perannya dalam serial televisi Sliders, Andrew Clements dalam My Secret Identity, Vern Tessio dalam film Stand by Me, Charlie C...

Family-founded supermarket chain in the United States D'Agostino SupermarketsFormerlyYorkville Food ShoppeD'Agostino BrothersCompany typePrivateIndustryGrocery retailFounded1932FoundersPasquale Patsy D'AgostinoNicola Nick D'AgostinoHeadquartersLarchmont, New York, United StatesNumber of locations26 stores (peak)[1]10 stores (2019)[2]Area servedNew York CityWestchester CountyKey peopleNicholas D'Agostino Jr., ChairmanNicholas D'Agostino III, CEOG. Robert James, PresidentRevenue...

Yang TerhormatN. RangaswamyN. Rangaswamy pada 2007 Ketua Menteri PuducherryPetahanaMulai menjabat 16 Mei 2011PendahuluV. VaithilingamPenggantiPetahanaDaerah pemilihanKadirkamamMasa jabatan1 October 2006 – 4 September 2008PendahuluPosisi ditetapkanPenggantiV. VaithilingamKetua Menteri PondicherryMasa jabatan27 October 2001 – 1 October 2006PendahuluP. ShanmugamPenggantiposisi dihapuskanDaerah pemilihanThattanchavady Informasi pribadiLahir04 Agustus 1950 (umur 73)P...

Kanon Pāli Vinaya Pitaka Sutta-vibhanga Khandhaka Pari-vara Sutta Pitaka ...

Cake Marble cakeTypeCakePlace of originGermanyMain ingredientsLight and dark batter Media: Marble cakeA marble cake (German: Marmorkuchen, pronounced [ˈmaʁmoːɐ̯ˌkuːxn̩] ⓘ), or Marmor (German: [ˈmaʁmoːɐ̯] ⓘlit. 'marble')) is a cake with a streaked or mottled appearance (like marble) achieved by very lightly blending light and dark batter.[1] Due to its zebra-striped pattern, it is also called zebra cake. It can be a mixture of vanilla an...

Interest rate taking inflation into account Yields on inflation-indexed government bonds of selected countries and maturities. The real interest rate is the rate of interest an investor, saver or lender receives (or expects to receive) after allowing for inflation. It can be described more formally by the Fisher equation, which states that the real interest rate is approximately the nominal interest rate minus the inflation rate. If, for example, an investor were able to lock in a 5% interest...

Independent city in Virginia, United StatesHampton, VirginiaIndependent cityHampton Downtown Historic District FlagSealMotto: From the Sea to the StarsLocation in the State of VirginiaHamptonLocation in VirginiaShow map of VirginiaHamptonLocation in the United StatesShow map of the United StatesCoordinates: 37°02′06″N 76°21′36″W / 37.034946°N 76.360126°W / 37.034946; -76.360126CountryUnited StatesStateVirginiaCountyNone (Independent city)Settled1610&#...

Blu-ray/DVD set collection of theatrical Tex Avery cartoons Tex Avery Screwball Classics is a series of single-disc Blu-ray and DVD sets by Warner Bros. Home Entertainment's Warner Archive unit collecting various theatrical cartoons from animation director Tex Avery during his tenure at the Metro-Goldwyn-Mayer studio's cartoon division between the years of 1942 and 1955. It is the first comprehensive collection of Avery's MGM shorts to be released on home media in North America since The Comp...

Doug BrochuDoug BrochuLahir29 September 1990 (umur 33)Fayetteville, North Carolina, United States[1]PekerjaanAktor, Komedian, pengisi suaraTahun aktif2007–2012 Douglas Doug Brochu (lahir 29 September 1990) adalah aktor, komedian dan aktor pengisi suara asal Amerika Serikat. Dia terkenal karena memerankan Grady Mitchell di Disney Channel Original Series, Sonny with a Chance. Karier Doug terkenal karena memerankan Grady Mitchell di Disney Channel Original Series, Sonny with...

Canadian ice hockey player (born 2004) Ice hockey player Matt Poitras Poitras with the Boston Bruins in 2023Born (2004-03-10) March 10, 2004 (age 20)Ajax, Ontario, CanadaHeight 5 ft 11 in (180 cm)Weight 172 lb (78 kg; 12 st 4 lb)Position CentreShoots RightNHL team Boston BruinsNHL draft 54th overall, 2022Boston BruinsPlaying career 2023–present Matthew Poitras (/ˈpɒtjrɑː/; born March 10, 2004) is a Canadian professional ice hockey centre for t...

Component city in Negros Occidental, Philippines Component city in Western Visayas, PhilippinesBagoComponent cityCity of BagoFrom top, left to right: Public Plaza, Balay ni Tan Juan, Buenos Aires Mountain Resort, Tan Juan Monument, Kipot Twin Falls. FlagSealNicknames: Home of Historical and Natural Treasures Boxing Capital of the Philippines Motto(s): Go, Bago!Map of Negros Occidental with Bago highlightedOpenStreetMapBagoLocation within the PhilippinesCoordinates: 10°32′20″N ...

Unilever PLCLogo Sede della Unilever a Londra Stato Regno Unito Forma societariaPublic company Borse valori Euronext: UNA LSE: ULVR NYSE: UN NYSE: UL ISINGB00B10RZP78 Fondazione1929 (fusione tra Margarine Unie e fratelli Lever)1966 (Unilever S.p.A., Italia) Fondata daWilliam Hesketh Lever, Anton Jan Jurgens, Samuel Van den Berg Sede principaleLondra e Rotterdam Persone chiave Nils Andersen (Chairman) Alan Jope (A.D.) SettoreProduzione beni di largo consumo ProdottiCibi, prodotti per la c...

此條目可能包含不适用或被曲解的引用资料,部分内容的准确性无法被证實。 (2023年1月5日)请协助校核其中的错误以改善这篇条目。详情请参见条目的讨论页。 各国相关 主題列表 索引 国内生产总值 石油储量 国防预算 武装部队(军事) 官方语言 人口統計 人口密度 生育率 出生率 死亡率 自杀率 谋杀率 失业率 储蓄率 识字率 出口额 进口额 煤产量 发电量 监禁率 死刑 国债 ...

Tommy LeeLee tahun 2012LahirThomas Lee Bass3 Oktober 1962 (umur 61)Athena, YunaniKebangsaanAmerika SerikatPekerjaanMusisiTahun aktif1979–sekarangSuami/istri Elaine Starchuk (m. 1984; c. 1985) Heather Locklear (m. 1986; c. 1993) Pamela Anderson (m. 1995; c. 1998) Brittany Furlan (m. 2019) ...

Historic house in New York, United States For the Governor of Missouri, see Guy Brasfield Park. United States historic placeGuy Park ManorU.S. National Register of Historic Places View from the Mohawk River side (2020)Show map of New YorkShow map of the United StatesLocationW. Main St.,Amsterdam, New YorkCoordinates42°56′49″N 74°12′36″W / 42.94694°N 74.21000°W / 42.94694; -74.21000Built1774Architectural styleGeorgianNRHP reference No.73001206...

Shorland redirects here. For other uses, see Shorland (disambiguation). This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Shorland armoured car – news · newspapers · books · scholar · JSTOR (December 2012) (Learn how and when to remove this message) Armoured car Shorland Internal Security Vehicle A Mk1 Shorlan...
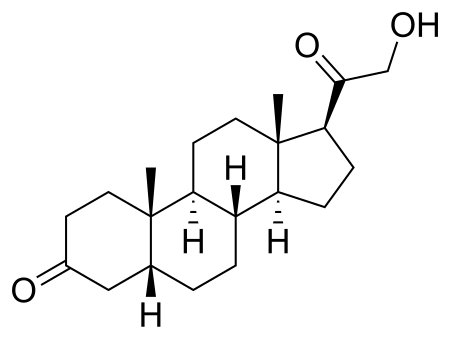
Chemical compound HydroxydioneClinical dataTrade namesViadril, Predion, PresurenOther names21-Hydroxy-5β-pregnane-3,20-dioneATC codeNoneLegal statusLegal status BR: Class C1 (Other controlled substances)[1] Identifiers IUPAC name (5R,8R,9S,10S,13S,14S,17S)-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-3-one CAS Number303-01-5PubChem CID257630DrugBankDB08956 YChemSpider226020UNIIB7VFN88375CompTox Dashboard (EPA)D...

