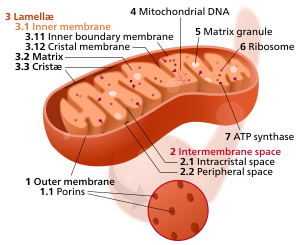
Crista
| |||||||
Read other articles:

This article is about the Vangelis album. For other albums, see Dragon (disambiguation) § Albums. This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (February 2011) (Learn how and when to remove this template message) 1978 studio album by VangelisThe DragonStudio album by VangelisReleased1978RecordedJune 1971StudioMarquee Studios, LondonGenreProgres...

Two-colored Cymbidium Cymbidium bicolor ssp. pubescens Klasifikasi ilmiah Kerajaan: Plantae (tanpa takson): Angiospermae (tanpa takson): Monokotil Ordo: Asparagales Famili: Orchidaceae Subfamili: Epidendroideae Tribus: Cymbidieae Subtribus: Cyrtopodiinae Alliance: Cymbidium Genus: Cymbidium Spesies: C. bicolor Nama binomial Cymbidium bicolor(L.) Sw. (1799) Subspecies Cymbidium bicolor. ssp. obtusum Du Puy & P.J.Cribb (1988) Synonyms: Cymbidium crassifolium Wall. (1832), Cymbidium ma...

2023 video game 2023 video gameHogwarts LegacyDeveloper(s)Avalanche SoftwarePublisher(s)Warner Bros. Games[a]Director(s)Alan TewProducer(s)James CabreraArtist(s)Jeff BunkerWriter(s)Adrian RoppMoira SquierGenese DavisCade JohnsonLoreen NariariShane LewisAdam KofordNatalie GrayComposer(s)Chuck E. MyersJ. Scott RakozyPeter MurrayAlexander HorowitzSeriesWizarding WorldEngineUnreal Engine 4Platform(s)PlayStation 5Windows 10Windows 11Xbox Series X/SPlayStation 4Xbox OneNintendo SwitchReleas...

Body of water in the Arctic Ocean from northeast of Greenland to Svalbard Not to be confused with the Weddell Sea. Wandel SeaWestern part of the Wandel SeaWandel SeaLocationArctic OceanCoordinates82°15′N 17°00′W / 82.250°N 17.000°W / 82.250; -17.000TypeSeaBasin countriesGreenland and NorwayMax. length800 km (500 mi)References[1] The Wandel Sea (Danish: Wandelhavet; also known as McKinley Sea[2]) is a body of water in the Arctic Oc...

Roman villa near Loures, Portugal Roman Ruins of FrielasAlternative nameArcheological site of FrielasLocationFrielas, Loures, Lisbon district, Greater Lisbon, PortugalCoordinates38°49′37″N 9°08′38″W / 38.8268747°N 9.1438332°W / 38.8268747; -9.1438332TypeRuinsHistoryCulturesRoman EmpireSite notesArchaeologistsunknownOwnershipPortuguese RepublicPublic accessPrivate, Frielas The Roman Villa of Frielas is located in the parish of Frielas in the munici...

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Ostrołęka Voivodeship – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this template message) Ostroleka Voivodeship Ostrołęka Voivodeship (Polish: województwo ostrołęckie) was a unit of administrative division and local governmen...

У этого термина существуют и другие значения, см. Украинский легион. Украинские сечевые стрельцыСечевые украинские стрельцы (СУС[1])укр. Українські січові стрільці Флаг УСС (1914) Годы существования 1914—1920 Страна Австро-Венгрия ЗУНР УНР Подчинение Вооруженные �...

Цыгане в Чехии — этническое меньшинство, проживающее в Чехии. При проведении переписи 2011 года 13 150 человек назвали себя цыганами, но эксперты полагали, что в республике проживают 240 300 этнических цыган[1]. История Первое предполагаемое свидетельство о цыганах в Чехии о...

Fourth-largest moon of Uranus ArielAriel in greyscale as imaged by Voyager 2 in 1986. Extensive grabens are visible, including the Kachina Chasmata in the upper part of the image.DiscoveryDiscovered byWilliam LassellDiscovery date24 October 1851DesignationsDesignationUranus IPronunciation/ˈɛəriəl/ or /ˈæriəl/[1]AdjectivesArielian /æriˈiːliən/[2]Orbital characteristics[3]Semi-major axis190900 kmEccentricity0.0012Orbital period (sidereal)2.520&#...

Defunct political party in the Netherlands RSAP redirects here. For the cryptography task, see RSA problem. For the bluetooth profile, see Remote SIM Access Profile. Revolutionary Socialist Party Revolutionair Socialistische PartijLeaderHenk SneevlietChairmanPiet J. Schmidt (RSAP)Founded1929Dissolved1940Split fromCommunist Party HollandPreceded bySocialist PartySucceeded byMarx–Lenin–Luxemburg Front (clandestine)HeadquartersAmsterdamNewspaperDe Baanbreker (1929-1935)De Nieu...

Artikel ini bukan mengenai Pamungkas, Sri Bintang Pamungkas, atau Bambang Pamungkas. Ge PamungkasLahirGenrifinadi Pamungkas25 Januari 1989 (umur 35)Jakarta, IndonesiaAlmamaterUniversitas Katolik ParahyanganPekerjaanAktorpelawak tunggalTahun aktif2012—sekarangSuami/istriAnastasia Herzigova (m. 2018) Genrifinadi Pamungkas (lahir 25 Januari 1989) adalah pelawak tunggal dan aktor berkebangsaan Indonesia.[1] Karier Nama Ge mulai dikenal setelah me...

Logo Another World Another World, juga dikenal sebagai Out of This World di Amerika Utara dan Outer World[a] di Jepang, adalah sebuah permainan aksi-petualangan tahun 1991 rancangan Éric Chahi untuk Delphine Software. Permainan tersebut mengisahkan cerita Lester, seorang ilmuwan muda yang, akibat sebuah eksperimen yang berakhir buruk, menemukan dirinya sendiri berada di sebuah dunia makhluk asing berbahaya dimana ia terpaksa untuk bertarung untuk bertahan hidup. Referensi Catatan ^ O...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Januari 2023. Murphys Hotel, ketika masih dikenal sebagai Mitchler Hotel. Murphys Hotel di Murphys, California merupakan salah satu hotel tertua yang masih beroperasi di California. Pertama kali bernama Sperry and Perry Hotel, bangunan ini dibuka oleh James L. Sperr...

هاري هولكيري (بالفنلندية: Harri Hermanni Holkeri) معلومات شخصية اسم الولادة (بالفنلندية: Harri Hermanni Holkeri) الميلاد 6 يناير 1937(1937-01-06)أوريبان الوفاة 7 أغسطس 2011 (74 سنة)هلسنكي مكان الدفن مقبرة هييتانييمي[1] مواطنة فنلندا مناصب عضو برلمان فنلندا عضو خلال الفترة23 مارس ...

Irish stew with no fixed recipe, built around boiled sausages CoddleCoddleAlternative namesDublin coddlePlace of originIrelandMain ingredientsPotatoes, pork sausage, rashers, onion Coddle (sometimes Dublin coddle; Irish: cadal)[1] is an Irish dish which is often made to use up leftovers. It most commonly consists of layers of roughly sliced pork sausages and rashers (thinly sliced, somewhat-fatty back bacon) with chunky potatoes, sliced onion, salt, pepper, and herbs. Traditionally, i...

This article needs to be updated. Please help update this article to reflect recent events or newly available information. (September 2022) Overview of solar power in the U.S. state of New York Installing rooftop solar panels in PoughkeepsieNew York has a renewable portfolio standard of 30% from renewable sources by 2015. In 2015 24% was renewable, 6% short of the goal. Wind is the predominant generating technology.[1] In 2018, the New York State Energy Research and Development Autho...

地獄第19層Naraka 19電影DVD封面基本资料导演黎妙雪监制禤嘉珍制片張珮詩、禤志傑编剧黎妙雪陳十三原著蔡駿小說《地獄的十九層》主演鍾欣潼泳 兒譚俊彥譚耀文李曼筠冼色麗蔡卓妍配乐黎允文主題曲《愛的凶間》/冼色麗摄影蔡崇輝剪辑葉婉婷制片商天下影畫有限公司片长98分鐘产地 香港语言粵語上映及发行上映日期 2007年8月28日 (2007-08-28) 2007年9月6日 (2007-09-0...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Cet article ne cite pas suffisamment ses sources (avril 2019). Si vous disposez d'ouvrages ou d'articles de référence ou si vous connaissez des sites web de qualité traitant du thème abordé ici, merci de compléter l'article en donnant les références utiles à sa vérifiabilité et en les liant à la section « Notes et références ». En pratique : Quelles sources sont attendues ? Com...

关于与「郝建秀」標題相近或相同的条目页,請見「郝建秀 (企业家)」。 郝建秀 郝建秀(1935年11月—),山东青岛人,中华人民共和国政治人物。原华东纺织工学院纺织工程系毕业,研究员级高级工程师。曾是纺织工人,在工作中独创了一套细纱工作法,被评为全国纺织工业系统劳动模范;后受到中共培养,转而从政。1954年5月加入中国共产党,是中共第十一至第�...

経済的、社会的及び文化的権利に関する国際規約の選択議定書 締約国 署名・未批准国 未署名・未締約国通称・略称 社会権規約選択議定書起草 1993年-2008年署名 2008年12月10日、国際連合総会(ニューヨーク国際連合本部)において採択。2009年9月24日署名のため開放。署名場所 ニューヨーク発効 2013年5月5日寄託者 国際連合事務総長言語 アラビ�...