Cerliponase alfa
| |||||||||||||||||||||||||||||||||||||
Read other articles:

American country band This article is about the American country band. For other uses, see The Chicks (disambiguation). The ChicksThe Dixie Chicks in Austin, Texas, in 2006Background informationAlso known asDixie ChicksOriginDallas, Texas, U.S.Genres Country[1] bluegrass[1] country pop[1] Years active1989–presentLabels Crystal Clear Sound Monument Nashville Columbia Nashville SpinoffsCourt Yard HoundsMembers Emily Strayer Martie Maguire Natalie Maines Past members Ro...

Latvijas Radio Amatieru LīgaLatvian Amateur Radio LeagueAbbreviationLRALTypeNon-profit organizationPurposeAdvocacy, EducationLocationRiga, Latvia KO26bwRegion served LatviaOfficial language LatvianChairmanImants Tukleris YL3GCTAffiliationsInternational Amateur Radio UnionWebsitehttp://www.lral.lv/ The Latvijas Radio Amatieru Līga (LRAL) (in English, Latvian Amateur Radio League) is a national non-profit organization for amateur radio enthusiasts in Latvia. Key membership benefits of...

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Cet article ne cite pas suffisamment ses sources (juin 2017). Si vous disposez d'ouvrages ou d'articles de référence ou si vous connaissez des sites web de qualité traitant du thème abordé ici, merci de compléter l'article en donnant les références utiles à sa vérifiabilité et en les liant à la section « Notes et références » En pratique : Quelles sources sont attendues ? Comme...

ماجد هزازي معلومات شخصية الاسم الكامل ماجد هزازي الميلاد 1 يوليو 1988 (العمر 35 سنة)الرياض الطول 172 سم مركز اللعب ظهير أيمن الجنسية السعودية معلومات النادي النادي الحالي نادي الشعله الرقم 88 المسيرة الاحترافية1 سنوات فريق م. (هـ.) 1 عدد مرات الظهور مع الأندية وعدد الأهداف تحسب...

Gábor Pogány (Budapest, 28 ottobre 1915 – Roma, 30 ottobre 1999) è stato un direttore della fotografia ungherese naturalizzato italiano. Indice 1 Biografia 2 Filmografia 3 Bibliografia 4 Altri progetti 5 Collegamenti esterni Biografia Nato in Ungheria a Budapest, frequenta la facoltà di Architettura della sua città. Dopo essersi laureato, si trasferisce in Inghilterra, occupandosi di fotografia legata al cinema sino a diventare aiuto operatore. Alla fine degli anni trenta arriva a Roma...

12th-century Bishop of London-elect Anselm of St SabaBishop of London electElectedabout 22 March 1136Installed1137Term ended1138PredecessorGilbert UniversalisSuccessorRobert de SigelloOther post(s)Abbot of Bury St. EdmundsOrdersConsecrationnever consecratedPersonal detailsDied3 January 1148DenominationCatholic Anselm[a] (died 1148) was a medieval bishop of London whose election was quashed by Pope Innocent II. He was a monk of Chiusa, abbot of Saint Saba in Rome, papal legate to ...

Penyuntingan Artikel oleh pengguna baru atau anonim untuk saat ini tidak diizinkan.Lihat kebijakan pelindungan dan log pelindungan untuk informasi selengkapnya. Jika Anda tidak dapat menyunting Artikel ini dan Anda ingin melakukannya, Anda dapat memohon permintaan penyuntingan, diskusikan perubahan yang ingin dilakukan di halaman pembicaraan, memohon untuk melepaskan pelindungan, masuk, atau buatlah sebuah akun. Adolf HitlerPotret resmi, 1938 Führer JermanMasa jabatan2 Agustus 1934 –&...
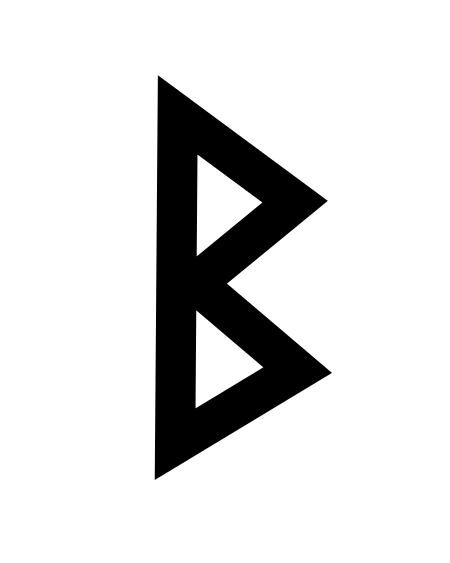
此條目介紹的是拉丁字母中的第2个字母。关于其他用法,请见「B (消歧义)」。 提示:此条目页的主题不是希腊字母Β、西里尔字母В、Б、Ъ、Ь或德语字母ẞ、ß。 BB b(见下)用法書寫系統拉丁字母英文字母ISO基本拉丁字母(英语:ISO basic Latin alphabet)类型全音素文字相关所属語言拉丁语读音方法 [b][p][ɓ](适应变体)Unicode编码U+0042, U+0062字母顺位2数值 2歷史發...

Galatama IVMusim1983-1984Tanggal30 November 1983 s/d 20 Mei 1984JuaraYanita UtamaJumlah pertandingan347Jumlah gol444 (1,28 per pertandingan)Pencetak golterbanyak Bambang Nurdiansyah (13 gol)(Yanita Utama)← 1982-83 1984 → Galatama 1983-84 atau juga disebut Galatama IV adalah kompetisi Galatama musim ke 4. Ikhtisar Pada Galatama musim ini banyak perubahan-perubahan peraturan yang dibuat oleh PSSI diantaranya adalah. Larangan penggunaan pemain asing. Divisi I musim sebelumnya tidak ada ...

Raja Ramanna Centre for Advanced TechnologyAbbreviationRRCATFormation19 February 1984 (1984-02-19)PurposePhysicsHeadquartersIndore, Madhya PradeshLocationIndiaCoordinates22°40′01″N 75°48′32″E / 22.667°N 75.809°E / 22.667; 75.809Parent organisationDepartment of Atomic EnergyWebsitewww.rrcat.gov.in The Raja Ramanna Centre for Advanced Technology is a unit of Department of Atomic Energy, Government of India, engaged in R&D in non-nuclear fr...

Ritratto di Algernon Sidney. Algernon Sidney (Castello di Baynard, 14 o 15 gennaio 1623 – 7 dicembre 1683) è stato un politico e nobile inglese. Teorico repubblicano, si oppose a Carlo II d'Inghilterra e rimase coinvolto in un complotto contro il re finendo giustiziato per tradimento. Indice 1 Biografia 2 Guerra civile inglese 3 Ambasciatore baltico 4 Esilio 5 Contro la monarchia 6 Processo ed esecuzione 7 Ascendenza 8 Opere 9 Note 10 Bibliografia 11 Voci correlate 12 Altri progetti 13 Col...

1948 film Private BomDirected byLars-Eric KjellgrenWritten byPaul Baudisch Nils Poppe Adolf SchützProduced byHarald MolanderStarringNils Poppe Inga Landgré Gunnar BjörnstrandCinematographyGunnar FischerEdited byOscar RosanderMusic byKai Gullmar Sune WaldimirProductioncompanyFribergs Filmbyrå ABDistributed byFribergs FilmbyråRelease date 15 December 1948 (1948-12-15) Running time89 minutesCountrySwedenLanguageSwedish Private Bom (Swedish: Soldat Bom) is a 1948 Swedish comed...

New PerspectiveLagu oleh Panic! at the Discodari album Jennifer's Body: Original Motion Picture SoundtrackDirilis28 Juli 2009FormatUnduhan digitalDirekam2009Genre Pop punk power pop alternative rock pop rock Durasi3:47Label Fueled by Ramen Decaydance Pencipta Brendon Urie, John Feldman Produser Brendon Urie John Feldmann New Perspective adalah lagu milik grup musik rock asal Amerika Serikat, Panic! at the Disco yang dirilis pada 28 Juli 2009 sebagai singel promosi dari film Jennifer's Body. V...

安倍晋太郎安倍晋太郎(攝於1987年4月21日) 日本第112、113任外務大臣任期1982年11月27日—1986年7月22日总理中曾根康弘前任櫻内義雄继任倉成正 日本第42任通商產業大臣任期1981年11月30日—1982年11月27日总理鈴木善幸前任田中六助(日语:田中六助)继任山中貞則 日本第41任内閣官房長官任期1977年11月28日—1978年12月7日总理福田赳夫前任園田直继任田中六助(日语�...

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: 1999 Indian general election in Haryana – news · newspapers · books · scholar · JSTOR (May 2019) (Learn how and when to remove this message) 1999 Indian general election in Haryana← 19982004 →10 seats First party Second party Third party Party...

История теории вероятностей отмечена многими уникальными особенностями. Прежде всего, в отличие от появившихся примерно в то же время других разделов математики (например, математического анализа или аналитической геометрии), у теории вероятностей по существу не было ...

Map of Uzbekistan History of Uzbekistan Prehistory Teshik-Tash 1 Scythian Early history Sogdia Transoxiana Bactria Medieval history Turkic Khaganate Onoq Türgesh Tang Protectorate Muslim conquest Umayyad Caliphate Abbasid Caliphate Samanid Empire Ghaznavids Kara-Khanid Khanate Anushtegin dynasty Khwarazmian Empire Mongol conquest Chagatai Timurid Empire Uzbek Khanate Modern period Russian period Emirate of Bukhara Khorezm People's Soviet Republic Bukharan People's Soviet Republic Uzbek Sovi...

Defunct American public company aQuantiveWebsitewww.aquantive.com (offline) aQuantive, Inc. was the parent company of a group of three digital marketing service and technology companies: Avenue A/Razorfish, Atlas Solutions, and DRIVE Performance Solutions. Based in Seattle, Washington, the company was founded in 1997. According to Advertising Age magazine, in 2005 it ranked 14th by revenue among advertising agencies worldwide. On May 18, 2007, Microsoft announced that it would acquire the com...

丹尼斯·德米特里耶夫摄于2015年個人資料全名丹尼斯·谢尔盖耶维奇·德米特里耶夫出生 (1986-03-23) 1986年3月23日(38歲) 苏联梁赞州普龍斯克區特尔诺沃身高1.77米(5英尺10英寸)體重90公斤(200磅)車隊資料環境场地自行车 獎牌紀錄 男子自行车 代表 俄羅斯 奥林匹克运动会 2016 里约热内卢 个人竞速赛 世界場地自由車錦標賽 2017 香港 个人竞速赛 2013 明斯克 个人竞速�...

Годы 1894 · 1895 · 1896 · 1897 — 1898 — 1899 · 1900 · 1901 · 1902 Десятилетия 1870-е · 1880-е — 1890-е — 1900-е · 1910-е Века XVIII век — XIX век — XX век 2-е тысячелетие XVII век XVIII век XIX век XX век XXI век 1790-е 1790 1791 1792 1793 1794 1795 1796 1797 1798 1799 1800-е 1800 1801 1802 1803 1804 1805 1806 1807 1808 1809 1810-е 1810 1811 1812 1813 1814 1815 1816...

