Read other articles:

Part of Barcelona's commuter rail service R1 RG1 A 447 Series train on a R1 service at Malgrat de Mar railway station in 2013.OverviewService typeCommuter railStatusOperationalLocaleBarcelona metropolitan area and province of GironaFirst service R1: 1989 (1989) RG1: 24 March 2014 (2014-03-24) Current operator(s)Renfe OperadoraRidership102,214 (2008)[1][a]Annual ridership28 million (2016)[2]RouteTermini R1: Molins de Rei RG1: L'Hospitalet de Llo...

His New JobPoster rilis teatrikalSutradaraCharlie ChaplinProduserJess RobbinsDitulis olehCharlie ChaplinLouella Parsons[1]PemeranCharles ChaplinBen TurpinCharlotte MineauLeo WhiteRobert BolderCharles J. StineArthur W. BatesJess RobbinsPenyuntingBret HamptonCharlie ChaplinDistributorEssanay StudiosTanggal rilis 01 Februari 1915 (1915-02-01) Durasi32 menitNegaraAmerika SerikatBahasaBisuIntertitel Inggris Film lengkap His New Job adalah sebuah film komedi bisu pendek Amerika 1915 ya...

Pour les articles homonymes, voir Louis II. Ne doit pas être confondu avec Louis Le Bègue Duportail. Louis II Le couronnement de Louis II,enluminure du XIVe siècle. Titre Roi des Francs(Francie occidentale) 6 octobre 877 – 11 avril 879(1 an, 6 mois et 5 jours) Couronnement 8 décembre 877 à Compiègne7 septembre 878 à Troyes Prédécesseur Charles II Successeur Louis III et Carloman II Biographie Titre complet Roi de Francie occidental...
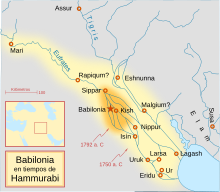
Relieve Reina de la Noche (ca. 1800 a. C.) El altorrelieve La Reina de la Noche, también llamado Relieve Burney, datado entre los años 1800-1750 a. C., fue esculpido en época del imperio paleobabilónico, bajo el reinado de Hammurabi, que se extendió por Mesopotamia en la zona de los ríos Tigris y Éufrates, actual Irak.[1][2] Historia La pieza procede del sur de Irak, y llegó a Inglaterra en 1924 donde permaneció en manos privadas hasta que fue adquirida por el...

Leafy vegetable dish from Indian subcontinent For the footballer, see Kaimar Saag. For the medical term, see Serum-ascites albumin gradient. SaagSarson ka saag with makki ki rotiAlternative namesSaaga or tuna (Odisha), shaag, shaak, saagwalaRegion or statePunjabMain ingredientsVarious kinds of edible plants Media: Saag Saag (Hindustani: [ˈsɑːg]), also spelled sag or saga, is an Indian subcontinental leafy vegetable dish eaten with bread such as roti or naan,[1] ...

Public park in Manhattan, New York This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Abingdon Square Park – news · newspapers · books · scholar · JSTOR (January 2023) (Learn how and when to remove this message) South entrance Abingdon Square Park is located in the New York City borough of Manhattan in Greenwic...
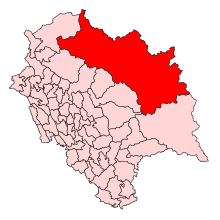
Legislative Assembly constituency in Himachal Pradesh State, India Lahaul and SpitiConstituency No. 21 for the Himachal Pradesh Legislative AssemblyConstituency detailsCountryIndiaRegionNorth IndiaStateHimachal PradeshDistrictLahaul and SpitiLS constituencyMandiEstablished1967Total electors25,496[2]ReservationSTMember of Legislative Assembly14th Himachal Pradesh Legislative AssemblyIncumbent Vacant[1] PartyTBAElected yearTBA Lahaul and Spiti Assembly constituency is one of the...

尊敬的拿督赛夫丁阿都拉Saifuddin bin Abdullah国会议员馬來西亞国会下议院英迪拉马哥打现任就任日期2018年7月16日 (2018-07-16)前任法兹阿都拉曼(希盟公正党)多数票10,950(2018) 马来西亚外交部长任期2021年8月30日—2022年11月24日君主最高元首苏丹阿都拉首相依斯迈沙比里副职卡玛鲁丁查化(国盟土团党)前任希山慕丁(国阵巫统)继任赞比里(国阵巫统)任期2018年7月2�...

American dish of pasta and fresh vegetables Pasta primaveraPasta primavera: tagliatelle with broad beans, asparagus and peasCoursePastaPlace of origin Canada United States [1]Region or stateYarmouth County, Nova ScotiaMain ingredientsPasta, vegetables, soffritto (garlic, carrot, celery, olive oil) Media: Pasta primavera Pasta primavera (spring Pasta in Italian) is an American dish that consists of pasta in a cream sauce and fresh vegetables, invented in the 1970s.[2]...

Australian politician The HonourableSir John SeeKCMG JP14th Premier of New South WalesIn office28 March 1901 – 14 June 1904Preceded byWilliam LyneSucceeded byThomas WaddellConstituencyGrafton Personal detailsBorn(1844-10-14)14 October 1844Yelling, Huntingdonshire, EnglandDied31 January 1907(1907-01-31) (aged 62)Randwick, New South Wales, AustraliaSpouseCharlotte Mary Matthews (1876–1904)Children Charlotte I. A. (m. Hordern) John Charles Matthews Ruby Edith S. Percy Georg...

History United States NameUSS LST-566 BuilderMissouri Valley Bridge and Iron Company, Evansville, Indiana Laid down17 March 1944 Launched11 May 1944 Sponsored byMrs. George C. Martin Commissioned29 May 1944 Decommissioned15 March 1946 In servicewith Military Sea Transportation Service and Military Sealift Command as USNS LST-566 (T-LST-566) 1952-1973 Fate Transferred to Philippines, 13 September 1976 Stricken1 November 1973 Philippines NameBPR Lanao Del Norte (LT-504) Acquired1976 Decommissi...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: SMA Negeri 16 Jakarta – berita · surat kabar · buku · cendekiawan · JSTOR SMA Negeri 16 JakartaInformasiJumlah kelas21, (X, XI, XII)Jurusan atau peminatanIPA dan IPSRentang kelasX, XI IPA, XI IPS, X...

هذه المقالة بحاجة لصندوق معلومات. فضلًا ساعد في تحسين هذه المقالة بإضافة صندوق معلومات مخصص إليها. جزء من سلسلة مقالات حولالشيعة العقيدة توحيد الله الإيمان بالملائكة الإيمان بالكتب السماوية الإيمان بالرسل والأنبياء الإيمان باليوم الآخر الإيمان بالقضاء والقدر إحياء شهر �...

Частина серії проФілософіяLeft to right: Plato, Kant, Nietzsche, Buddha, Confucius, AverroesПлатонКантНіцшеБуддаКонфуційАверроес Філософи Епістемологи Естетики Етики Логіки Метафізики Соціально-політичні філософи Традиції Аналітична Арістотелівська Африканська Близькосхідна іранська Буддій�...

Species of fern in the family Psilotaceae Psilotum nudum Conservation status Least Concern (IUCN 3.1)[1] Scientific classification Kingdom: Plantae Clade: Tracheophytes Division: Polypodiophyta Class: Polypodiopsida Order: Psilotales Family: Psilotaceae Genus: Psilotum Species: P. nudum Binomial name Psilotum nudum(L.) P.Beauv.[2] Synonyms[2] Bernhardia antillarum K.Mull. Bernhardia capensis K.Mull. Bernhardia deppeana K.Mull. Bernhardia dichotoma Willd. ex ...

Fuerza de Protección de las Naciones Unidas - Sector Oeste United Nations Protection Force - Sector West Barra de UNPROFORActiva 7 de abril de 1992 a 31 de marzo de 1995 (2 años, 11 meses y 24 días).País CroaciaFidelidad Naciones UnidasTipo Fuerzas de paz de las Naciones UnidasFunción Asegurar la desmilitarización de su sector asignado en Eslavonia Occidental para contribuir a su pacificación que contribuyan con las negociaciones de paz a cargo de Naciones Unidas y...

هيكلية بيانات ويكيبيديا نطاقات ويكيبيديا نطاقات المهام نطاقات النقاش 0 (رئيسي/مقالة) نقاش 1 2 مستخدم نقاش المستخدم 3 4 ويكيبيديا نقاش ويكيبيديا 5 6 ملف نقاش الملف 7 8 ميدياويكي نقاش ميدياويكي 9 10 قالب نقاش القالب 11 12 مساعدة نقاش المساعدة 13 14 تصنيف نقاش التصنيف 15 100 بوابة نقاش الب�...

Deiningen Lambang kebesaranLetak Deiningen di Donau-Ries NegaraJermanNegara bagianBayernWilayahSchwabenKreisDonau-RiesPemerintahan • MayorKarl-Heinz StipplerLuas • Total15,32 km2 (592 sq mi)Ketinggian420 m (1,380 ft)Populasi (2013-12-31)[1] • Total1.898 • Kepadatan1,2/km2 (3,2/sq mi)Zona waktuWET/WMPET (UTC+1/+2)Kode pos86738Kode area telepon09081Pelat kendaraanDONSitus webwww.deiningen.de Deiningen ada...

Maisons typiques de Santana Maison d'un particulier (2008) Localisation Situation Santana, São Vicente Madère Portugal Architecture Style Vernaculaire modifier Les maisons de Colmo ou maisons typiques de Santana (en portugais : Casas típicas de Santana) sont le plus souvent situées entre la commune de Santana et la commune de São Vicente. Ces maisons triangulaires qui présentent un type d'architecture vernaculaire, font partie des lieux les plus visités sur l'île de Madèr...

Fictitious conspiracy causing anti-Catholic hysteria affecting England, Scotland, and Ireland Not to be confused with Gunpowder Plot. The execution of the five Jesuits The Popish Plot was a fictitious conspiracy invented by Titus Oates that between 1678 and 1681 gripped the kingdoms of England and Scotland in anti-Catholic hysteria.[1] Oates alleged that there was an extensive Catholic conspiracy to assassinate Charles II, accusations that led to the executions of at least 22 men...