Methylcrotonyl-CoA carboxylase
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Galeri King's Library di British Museum. King's Library (Perpustakaan Raja) adalah salah satu koleksi berbagai buku penting dari Zaman Pencerahan.[1] Awalnya dikumpulkan oleh Raja George III, perpustakaan ilmiah pribadi dengan koleksi lebih dari 65.000 buku ini kemudian diberikan kepada rakyat Britania Raya oleh Raja George IV. Koleksi buku-buku tersebut ditempatkan di sebuah galeri yang dibangun secara khusus di dalam British Museum dari tahun 1827 hingga 1997 dan sekarang menjadi ba...

Perjanjian RastattPeta Eropa setelah penandatanganan Perjanjian Utrecht, Rastatt, dan BadenKonteks Mengakhiri Perang Pewaris Spanyol Ditandatangani7 Maret 1714 (1714-03-07)LokasiRastatt, Markgrafschaft Baden-BadenPerunding Claude Louis, Adipati Villars Pangeran Eugene dari Savoia Pihak Kerajaan Prancis Habsburg Monarchy (Austria) BahasaPrancis Perjanjian Rastatt adalah sebuah perjanjian perdamaian yang ditandatangani oleh Kerajaan Prancis dan Austria pada tanggal 7 Maret 1714...

U.S. federal government business loan program Not to be confused with Public–private partnership, also often abbreviated as PPP. President Trump signs the Paycheck Protection Program and Health Care Enhancement Act (H.R. 266), April 24, 2020 The Paycheck Protection Program (PPP) is a $953-billion business loan program established by the United States federal government during the Trump administration in 2020 through the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) to help ...

Concours Eurovision de la chanson 1999 Dates Finale 29 mai 1999 Retransmission Lieu International Convention CentreJérusalem, Israël Présentateur(s) Dafna DekelYigal Ravid Sigal Shachmon Superviseur exécutif Christine Marchal-Ortiz Télédiffuseur hôte IBA Ouverture Vues de Jérusalem Entracte Dror Yikrah et Free par Dana International Participants Nombre de participants 23 Débuts Aucun Retour Autriche Bosnie-Herzégovine Danemark Islande Lituanie Relégation Finlande Grèce Macédoine...

Salah satu awak kabin Austrian Airlines mengarahkan penumpang ke tempat duduknya Awak kabin, juga dikenal sebagai pramugara (untuk pria) dan pramugari (untuk wanita) atau air host/air hostess, adalah bagian dari awak pesawat dalam penerbangan komersial, dalam banyak pesawat jet bisnis (business jets), dan beberapa pesawat udara milik pemerintahan.[1][2] Secara kolektif, disebut awak kabin, yang tanggung jawab utamanya adalah keselamatan dan kenyamanan penumpang. Kepramugaraan ...

Norman Fucking Rockwellsingolo discograficoScreenshot tratto dal video del branoArtistaLana Del Rey Pubblicazione1º novembre 2019 Durata4:09 Album di provenienzaNorman Fucking Rockwell! GenerePop EtichettaPolydor, Interscope ProduttoreLana Del Rey, Jack Antonoff RegistrazioneConway Recording Studios (Los Angeles) e House of Breaking Glass (Seattle) FormatiDownload digitale, streaming CertificazioniDischi d'argento Regno Unito[1](vendite: 200 000+) Lana Del Rey - cronol...

Indian border guard for the Indo-Tibetan border This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Indo-Tibetan Border Police – news · newspapers · books · scholar · JSTOR (October 2014) (Learn how and when to remove this message) Indo-Tibetan Border PoliceEmblem of the Indo-Tibetan Border PoliceIndo-Tibetan Bo...

Vehicle frame designed to protect occupants in the event of a crash Rollcage redirects here. For the PlayStation and PC video game, see Rollcage (video game). Racecar roll cage inside a Suzuki Swift A roll cage is a specially engineered and constructed frame built in (or sometimes around, in which case it is known as an exo cage) the passenger compartment of a vehicle to protect its occupants from being injured or killed in an accident, particularly in the event of a rollover. Designs Unimog ...

Yitzhak Navon Presiden Israel ke-5Masa jabatan24 Mei 1978 – 5 Mei 1983Perdana MenteriMenachem BeginPendahuluEphraim KatzirPenggantiChaim Herzog Informasi pribadiLahir(1921-04-09)9 April 1921Yerusalem, Mandat Britania atas PalestinaMeninggal6 November 2015(2015-11-06) (umur 94)Yerusalem, IsraelKebangsaanIsraelPartai politikHaMa'arakhSuami/istriOfira Resnikov (1963–93)Miri Shafir (2008–15)Anak2ProfesiPengarangTanda tanganSunting kotak info • L • B Yitzhak R...

Main article: 1996 United States presidential election 1996 United States presidential election in New York ← 1992 November 5, 1996 2000 → Nominee Bill Clinton Bob Dole Ross Perot Party Democratic Republican Independence Alliance Liberal Parties Conservative Freedom Home state Arkansas Kansas Texas Running mate Al Gore Jack Kemp Pat Choate Electoral vote 33 0 0 Popular vote 3,756,177 1,933,492 503,458 Percentage 59.47% 30.61% 7.97% County resu...
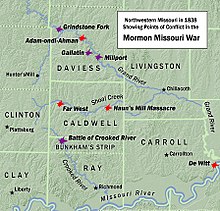
American politician (1809–1867) Sterling PricePrice in uniform c. 186211th Governor of MissouriIn officeJanuary 3, 1853 – January 5, 1857Preceded byAustin Augustus KingSucceeded byTrusten PolkMember of the U.S. House of Representativesfrom Missouri's at-large districtIn officeMarch 4, 1845 – August 12, 1846Preceded byJohn JamesonSucceeded byWilliam McDaniel Personal detailsBorn(1809-09-14)September 14, 1809Prince Edward County, Virginia, U.S.DiedSepte...

American television broadcasting company Weigel Broadcasting Co.Company typePrivateIndustryMediaFoundedJune 4, 1964; 60 years ago (1964-06-04)FounderJohn WeigelHeadquartersChicago, Illinois, United StatesKey people Norman Shapiro (Chairman & President) Neal Sabin (Vice Chairman & President of Content & Networks) Kyle Walker (Vice President of Technology) ProductsTelevision, BroadcastingOwner Norman Shapiro Websitewww.weigelbroadcasting.com Weigel Broadcasting Co....

آرتشويمعلومات عامةالموقع أرتشواي، لندن التقسيم الإداري إزلنغتون البلد المملكة المتحدة شبكة المواصلات مترو لندن المالك هيئة النقل في لندن الإدارة مترو لندن الخطوط Northern line (en) المحطات المجاورة هايغيت[1]على الخط: Northern line (en) باتجاه: هاي بارنيت، شرق ميل هيل — توفنيل ب�...

Castello-Molina di Fiemme komune di Italia Castello-Molina di Fiemme (it) Tempat Negara berdaulatItaliaDaerah otonom dengan status istimewaTrentino-Tirol SelatanProvinsi di ItaliaTrentino NegaraItalia PendudukTotal2.351 (2023 )GeografiLuas wilayah54,56 km² [convert: unit tak dikenal]Ketinggian900 m Berbatasan denganAltrei (en) Capriana Ville di Fiemme (en) Cavalese Pieve Tesino Telve Valfloriana SejarahSanto pelindungSanto George Organisasi politikAnggota dariAliansi Iklim (2003) ...

Illinois-based corporation that operates for-profit colleges This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Adtalem Global Education – news · newspapers · books ...

French government from 1804 to 1814 Ministers of NapoleonCabinet of the First French EmpireNapoleon IDate formed18 May 1804Date dissolved1 April 1814People and organisationsHead of stateNapoleonHead of governmentNapoleonHistoryPredecessorCabinet of the French ConsulateSuccessorProvisional Government of 1814 The First Cabinet of Napoleon I was appointed by the Emperor Napoleon I upon the establishment of the First French Empire on 18 May 1804, replacing the Cabinet of the Consulate. It was suc...

American defector to Nazi Germany Martin James MontiMonti listens to the U.S. Commissioner after being arrested at Mitchel Air Force Base, still in his uniform (January 1948)Born(1921-10-24)October 24, 1921St. Louis, Missouri, U.S.DiedSeptember 11, 2000(2000-09-11) (aged 78)Fort Lauderdale, Florida, U.S.Known forDefecting to Nazi Germany & the Waffen-SS in 1944Criminal statusDeceasedConviction(s)FederalTreason (18 U.S.C. § 2381) (21 counts)MilitaryDesertionTheftCriminal penalty...

American professional surfer (born 1992) John John John Alexander FlorenceFlorence at the 2013 Triple Crown of SurfingPersonal informationBorn (1992-10-18) October 18, 1992 (age 31)Honolulu, Hawai'iResidenceHale'iwa, Hawai'iHeight6 ft 1 in (185 cm)Weight185 lb (84 kg)Surfing careerBest year1st: 2016, 2017 - WSL World ChampionSponsorsMachu Picchu Energy, Stance, Nixon, VEIA Supplies, Clif Bar, Therabody, Yeti and Pyzel surfboardsMajor achievements 2x World Champio...

OradeaNagyvárad Ciudad y capital BanderaEscudo OradeaLocalización de Oradea en Rumanía Coordenadas 47°04′20″N 21°55′16″E / 47.072222222222, 21.921111111111Capital OradeaEntidad Ciudad y capital • País Rumania • Distrito BihorAlcalde Florin Birta (2021) (Partido Nacional Liberal (PN-L))Superficie • Total 111.2 km²Altitud • Media 142 m s. n. m.Población • Total 206 614 hab. • Dens...

Italian composer Cimarosa redirects here. Not to be confused with Cima Rossa. For the Italian actor, see Tano Cimarosa. This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (March 2022) (Learn how and when to remove this message) Engraving of Cimarosa by Luigi Rados Domenico Cimarosa (Italian: [doˈmeːniko tʃimaˈrɔːza] ⓘ; 17 December 1749 – 11 Janu...