Juglone
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Internet Protocol address (atau disingkat alamat IP) adalah label numerik yang ditetapkan untuk setiap perangkat yang terhubung ke jaringan komputer yang menggunakan Protokol Internet untuk komunikasi.[1][2] Alamat IP memiliki dua fungsi utama: host atau identifikasi antarmuka jaringan dan pengalamatan lokasi. Internet Protocol versi 4 (IPv4) mendefinisikan alamat IP sebagai nomor 32-bit.[2] Namun, karena pertumbuhan Internet dan menipisnya alamat IPv4 yang tersedia, v...

Archives Index 1, 2, 3, 4, 5 This page has archives. Sections older than 90 days may be automatically archived by Lowercase sigmabot III when more than 5 sections are present. If you're here because you want SuggestBot to send you suggestions, please see the instructions at the top of User:SuggestBot. We offer both subscription services and the possibility of receiving a single set of suggestions. Feedback on recommendations, ideas for new features, questions about how SuggestBot works, or a...

Istana Odawara 小田原城 Odawara, Prefektur Kanagawa, Jepang Istana Odawara Jenis Istana Jepang gaya Hirayama Dibangun 1447, dibangun ulang 1633, 1706 Digunakan Zaman Edo Diruntuhkan 1872 Dibukauntuk umum ya Pertempuran/Perang Pengepungan Odawara (1561)Pengepungan Odawara (1569)Pengepungan Odawara (1590) Istana Odawara (小田原城code: ja is deprecated , Odawara-jō) adalah istana yang terletak di Kota Odawara, Prefektur Kanagawa, Jepang. Sejarah Odawara dahulu merupakan benteng Kl...

Bola basket pada OLimpiade Musim Panas 2024LokasiStadion Pierre Mauroy (tahap pendahuluan 5×5)Arena Bercy (tahap final 5×5)Place de la Concorde (3×3)Tanggal27 Juli – 11 Agustus 2024Jumlah disiplin4Peserta352 dari 24 negara← 20202028 → Bola basket pada Olimpiade Musim Panas 2024 di Paris, Prancis akan diadakan dari 27 Juli hingga 11 Agustus 2024.[1] Pertandingan pendahuluan bola basket 5x5 akan berlangsung di Stadion Pierre Mauroy di Lille dan ...

Ernest Cline Información personalNacimiento 29 de marzo de 1972 (52 años)Ashland (Estados Unidos) Nacionalidad EstadounidenseReligión Ateísmo Lengua materna Inglés EducaciónEducado en Ashland High School Información profesionalOcupación Escritor Años activo desde 2009Género Ciencia ficción Obras notables Ready Player One Sitio web www.ernestcline.com [editar datos en Wikidata] Ernest Christy Cline (Ashland, Ohio; 29 de marzo de 1972) es un escritor de ciencia ficción, po...

Galileo di hadapan sidang Inkuisisi Roma Inkuisisi (dengan huruf I besar) adalah istilah yang secara luas digunakan untuk menyebut pengadilan terhadap ahli bidat oleh Gereja Katolik Roma. Istilah ini juga dapat bermakna tribunal gerejawi atau lembaga dalam Gereja Katolik Roma yang bertugas melawan atau menyingkirkan bidat, sejumlah gerakan ekspurgasi historis terhadap bidat (yang digiatkan oleh Gereja Katolik Roma), atau pengadilan atas seseorang yang didakwa bidat[1] Definisi dan tuj...

Masjid JamMasjid Abdurrahman PashaXhamia e Abdurrahman PashësXhamia me SahatMasjid Jam di Kota Peqin, AlbaniaAgamaAfiliasiIslamLokasiMunisipalitasPeqinNegaraAlbaniaKoordinat41°02′45″N 19°45′02″E / 41.04583°N 19.75056°E / 41.04583; 19.75056Koordinat: 41°02′45″N 19°45′02″E / 41.04583°N 19.75056°E / 41.04583; 19.75056ArsitekturGaya arsitekturArsitektur OttomanPeletakan batu pertama1666SpesifikasiKubah1Menara1 Masjid Jam (Xh...

Passiflora Passiflora 'Amethyst' Buah P. platyloba yang sering dirancukan dengan P. quadrangularis Klasifikasi ilmiah Kerajaan: Tumbuhan (tanpa takson): Tracheophyta (tanpa takson): Angiospermae (tanpa takson): Eudikotil (tanpa takson): Rosidae Ordo: Malpighiales Famili: Passifloraceae Genus: PassifloraL. Spesies Sekitar 500 spesies, lihat daftar spesies Passiflora Sinonim Anthactinia Bory ex M.Roem. Asephananthes Bory Astrophea Lam. ex M.Roem. Baldwinia Raf. Ceratosepalum Oerst. C...

ساندرو برتيني (بالإيطالية: Sandro Pertini) الرئيس السابع لجمهورية إيطاليا في المنصب9 يوليو 1978 – 29 يونيو 1985 رئيس الوزراء جوليو أندريوتيفرانشيسكو كوسيغاأرنالدو فورلاني جيوفاني سبادولينيأمنتوري فانفانيبتينو كراكسي جيوفاني ليوني فرانشيسكو كوسيغا مجلس النواب الإيط�...

US black power organization (1966–1982) Black panthers redirects here. For other uses, see Black panther (disambiguation). For similarly named organisations, see Black panther (disambiguation) § Political organisations. Black Panther Party AbbreviationBPPLeaderHuey P. NewtonFounded1966; 58 years ago (1966)Dissolved1982; 42 years ago (1982)HeadquartersOakland, CaliforniaNewspaperThe Black PantherMembershipc. 5,000 (1969)[1]Ideology Black nati...

North SomersetCounty constituencyfor the House of CommonsNorth Somerset in SomersetLocation of Somerset within EnglandCountySomersetElectorate78,469 (2018)[1]Major settlementsClevedon, Nailsea and PortisheadCurrent constituencyCreated2010Member of ParliamentLiam Fox (Conservative)SeatsOneCreated fromWoodspring1950–1983Created fromFrome and Weston-super-MareReplaced byWoodspring, Wansdyke and Wells[2]1885–1918Created fromEast SomersetReplaced byFrome and Weston-super-Mare ...

In order to facilitate organized, determined, and principled opposition to the wars, people have often founded anti-war organizations. These groups range from temporary coalitions which address one war or pending war, to more permanent structured organizations which work to end the concept of war and the factors which lead to large-scale destructive conflicts. The overwhelming majority do so in a nonviolent manner. The following list of anti-war organizations highlights past and present anti...

County in New York, United States Not to be confused with Schuyler, New York. County in New YorkSchuyler CountyCountySchuyler County Courthouse FlagSealLocation within the U.S. state of New YorkNew York's location within the U.S.Coordinates: 42°23′N 76°53′W / 42.39°N 76.88°W / 42.39; -76.88Country United StatesState New YorkFounded1854Named forPhilip SchuylerSeatWatkins GlenLargest communityWatkins GlenArea • Total342 sq mi (890&...

Questa voce o sezione sull'argomento Spagna è priva o carente di note e riferimenti bibliografici puntuali. Sebbene vi siano una bibliografia e/o dei collegamenti esterni, manca la contestualizzazione delle fonti con note a piè di pagina o altri riferimenti precisi che indichino puntualmente la provenienza delle informazioni. Puoi migliorare questa voce citando le fonti più precisamente. FallasNome originaleFalles Tipocittadina Periodo15-19 marzo Celebrata a Spagna / Valencia Al...
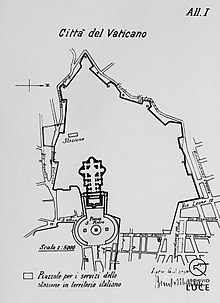
Confine tra la Città del Vaticano e l'ItaliaMappa della Città del VaticanoDati generaliStati Italia Città del Vaticano Lunghezza3,2 km Dati storiciIstituito nel1929 Causa istituzionePatti Lateranensi[1] Attuale dal1929 Causa tracciato attualePatti Lateranensi Manuale Il confine tra la Città del Vaticano e l'Italia descrive la linea di demarcazione tra questi due stati, avente una lunghezza di 3,2 km.[2] Stabilito dai Patti Lateranensi dell'11 febbraio 1929 e quin...

Jalur kargo Hachinohe RinkaiLokomotif DD56 menarik gerbong peti kemasIkhtisarJenisRel beratStatusAktifLokasiAomori, JepangTerminusTerminal kargo Hachinohe*KitanumaJalur penghubung Jalur Hachinohe Jalur Aoimori Penghubung sebelumnya Jalur cabang ke pos sinyal Jalur cabang ke Pelabuhan Hachinohe Stasiun2Layanan Kereta peti kemas Kereta angkutan kertas OperasiDibuka25 Maret 1966PemilikPemerintah daerah Prefektur AomoriOperatorHachinohe Rinkai TetsudōRangkaianLokomotif DD56Data teknisPanjang lin...

Imperial Roman legion Map of the Roman empire in AD 125, under emperor Hadrian, showing the Legio VIII Augusta, stationed on the river Rhine at Argentorate (Strasbourg, France), in Germania Superior province, from AD 75 to at least 371 Coin showing Claudius and Aquila of the V and VIII legions Aureus struck in 193 by Septimius Severus to celebrate VIII Augusta, one of the legions supporting his fight for purple. Legio VIII Augusta (Augustus' Eighth Legion) was one of the oldest legions of the...

В Википедии есть статьи о других людях с фамилией Хоссу. Катинка Хоссу Личная информация Пол женский Прозвище Железная леди Страна Венгрия Специализация плавание Клуб Team Iron[вд] Дата рождения 3 мая 1989(1989-05-03)[1][2] (35 лет) Место рождения Печ, Венгрия Рост 170 см Вес 56 �...

القوات الجوية الإكوادورية الدولة الإكوادور الإنشاء 1920 النوع سلاح الجو الدور حرب جوية الحجم 6389 فرد[1] جزء من القوات المسلحة الإكوادورية 72 طائرة الموقع الرسمي الموقع الرسمي الطـائرات هجومية كفير، أطلس شيتا طائرة تدريب إمبراير إي إم بي 314 سوبر توكانو طائرة نقل ...

Cet article est une ébauche concernant une localité chilienne. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Paillacoville et commune du Chili Héraldique Drapeau Administration Pays Chili Région Région des Fleuves Province Province de Valdivia Indicatif téléphonique +56 63 Démographie Gentilé Paillaquino/a Population 19 088 hab. (2012) Densité 21 hab./km2 Géographie Coordonnées 40°&...

