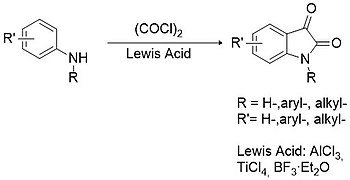
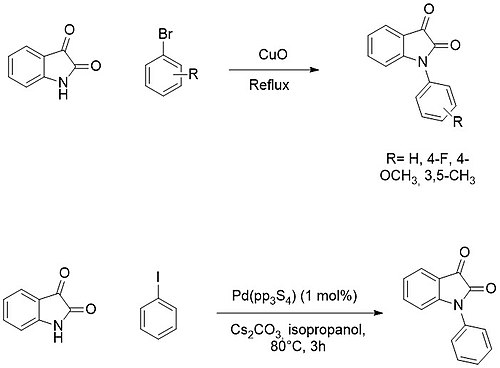
Isatin
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

العلاقات الأفغانية الجزائرية أفغانستان الجزائر أفغانستان الجزائر تعديل مصدري - تعديل العلاقات الأفغانية الجزائرية هي العلاقات الثنائية التي تجمع بين أفغانستان والجزائر.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين:...

Bonaire Boneirucode: pap is deprecated (Papiamento)Kota khusus dari BelandaBadan Publik Bonaire[1]Hotel Bellafonte Luxury Oceanfront BenderaLambang kebesaranHimne daerah: Tera di Solo y suave bientoLokasi Bonaire (lingkaran merah)di KaribiaKoordinat: 12°9′N 68°16′W / 12.150°N 68.267°W / 12.150; -68.267NegaraBelandaWilayah luar negeriBelanda KaribiaDimasukan ke dalam Belanda10 Oktober 2010 (Pembubaran Antillen Belanda)Ib...

هذه صفحة مساعدة لكيفية عمل شيء ما.تفصّل هذه الصفحة طرق أو إجراءات بعض جوانب قواعد وممارسات ويكيبيديا. هذه الصفحة ليست واحدة من سياسات أو إرشادات ويكيبيديا، حيث لم تفحص بدقة عبر المجتمع. في ويكيبيديا، لون الرابط يحدد حالته أي هل تمت زيارته من قبل أم أن الصفحة غير موجودة، وال�...

Julang Jambul-hitam Status konservasi Terancam (IUCN 3.1)[1] Klasifikasi ilmiah Kerajaan: Animalia Filum: Chordata Kelas: Aves Ordo: Bucerotiformes Famili: Bucerotidae Genus: Rhabdotorrhinus Spesies: R. corrugatus Nama binomial Rhabdotorrhinus corrugatus(Temminck, 1832) Julang Jambul-hitam (Rhabdotorrhinus corrugatus ) adalah spesies burung rangkong (famili Bucerotidae) yang dapat ditemukan di Thailand Selatan, Semenanjung Malaya, Sumatra, dan Kalimantan.[2] Julang ...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada November 2022. Dalam nama Tionghoa ini, nama keluarganya adalah Cao. Cao ZhongrongCao Zhongrong (kanan) lawan Eli BremerInformasi pribadiLahir3 November 1981 (umur 42)Tinggi18 m (59 ft 1⁄2 in)Berat73 kg (161 pon) (161 pon) Olahrag...

Questa voce o sezione sull'argomento Competizioni calcistiche non è ancora formattata secondo gli standard. Commento: Si invita a seguire il modello di voce Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Eredivisie 2010-2011 Competizione Eredivisie Sport Calcio Edizione 55ª Organizzatore Federazione calcistica dei Paesi Bassi Date dal 6 agosto 2010al 15 maggio 2011 Luogo Paesi Bassi Partecipanti 18 Risult...

ChellahSala ColoniaشالةBagian dari Chellah/Sala ColoniaLokasi di MarokoLokasiRabat, Rabat-Salé-Zemmour-Zaer, MarokoKoordinat34°00′24″N 06°49′13″W / 34.00667°N 6.82028°W / 34.00667; -6.82028Koordinat: 34°00′24″N 06°49′13″W / 34.00667°N 6.82028°W / 34.00667; -6.82028JenisPermukiman dan nekropolisSejarahDitinggalkan1154 Chellah (Arab: شالةcode: ar is deprecated ) atau Sala Colonia adalah nekropolis dan situs Romawi ...
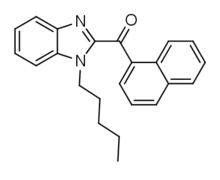
Chemical compound BIM-018Legal statusLegal status CA: Schedule II Identifiers IUPAC name 2-(naphthalene-1-carbonyl)-1-pentyl-1H-1,3-benzodiazole CAS Number2316839-70-8 YPubChem CID124519300ChemSpider29763750UNII2FNW3VE8P9Chemical and physical dataFormulaC23H22N2OMolar mass342.442 g·mol−13D model (JSmol)Interactive image SMILES CCCCCn1c2ccccc2nc1C(=O)c1cccc2c1cccc2 InChI InChI=1S/C23H22N2O/c1-2-3-8-16-25-21-15-7-6-14-20(21)24-23(25)22(26)19-13-9-11-17-10-4-5-12-18(17)19/h4-7,...

Jewish religion c. 516 BC–70 AD Part of a series onJudaism Movements Orthodox Haredi Hasidic Modern Conservative Conservadox Reform Karaite Reconstructionist Renewal Humanistic Haymanot Philosophy Principles of faith Kabbalah Messiah Ethics Chosenness God Names Musar movement Texts Tanakh Torah Nevi'im Ketuvim Ḥumash Siddur Piyutim Zohar Rabbinic Mishnah Talmud Midrash Tosefta Law Mishneh Torah Tur Shulchan Aruch Mishnah Berurah Aruch HaShulchan Kashrut Tzniut Tzed...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Countryside Agency – news · newspapers · books · scholar · JSTOR (March 2024) (Learn how and when to remove this message) Countryside AgencyPredecessorCountryside CommissionRural Development CommissionFounded1999Defunct2006SuccessorNatural EnglandCommission for...

Sindhi politician SaeenGhulam Murtaza Syedغلام مرتضي سيدG.M Syed in ceremony programMinister of Education of SindIn office18 March 1940 – 7 March 1941[1][2]PremierMir Bandeh Ali Khan TalpurGovernorLancelot GrahamHugh Dow Personal detailsBorn(1904-01-17)17 January 1904Sann, Bombay Presidency, British India(present-day Sindh, Pakistan)Died(1995-04-25)25 April 1995 (age 91)Karachi, Sindh, PakistanResting placeSann, SindhChildrenSyed Amir Hyder ShahSy...
Cet article est une ébauche concernant le jeu vidéo. Vous pouvez partager vos connaissances en l’améliorant (comment ?) (voir l’aide à la rédaction). Chronologies Chronologie du jeu vidéo 1983 1984 1985 1986 1987 1988 1989Décennies :1950 1960 1970 1980 1990 2000 2010 Chronologie dans le monde 1983 1984 1985 1986 1987 1988 1989Décennies :1950 1960 1970 1980 1990 2000 2010 Chronologies géographiques Afrique Afrique du ...

American popular music vocal group This article is about the band. For other uses, see Fifth Dimension (disambiguation). This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (April 2020) (Learn how and when to remove this message) The 5th DimensionThe 5th Dimension in 1969Back row: Townson and McLemore.Front row: LaRue, Davis, and McCoo.Background informationAlso k...

土库曼斯坦总统土库曼斯坦国徽土库曼斯坦总统旗現任谢尔达尔·别尔德穆哈梅多夫自2022年3月19日官邸阿什哈巴德总统府(Oguzkhan Presidential Palace)機關所在地阿什哈巴德任命者直接选举任期7年,可连选连任首任萨帕尔穆拉特·尼亚佐夫设立1991年10月27日 土库曼斯坦土库曼斯坦政府与政治 国家政府 土库曼斯坦宪法 国旗 国徽 国歌 立法機關(英语:National Council of Turkmenistan) ...

لمعانٍ أخرى، طالع جيمس برادلي (توضيح). جيمس برادلي معلومات شخصية الميلاد سنة 1954 (العمر 69–70 سنة) مواطنة الولايات المتحدة الأب جون برادلي الحياة العملية المهنة مؤرخ اللغات الإنجليزية تعديل مصدري - تعديل جيمس برادلي (بالإنجليزية: James Bradley) هو م...

Jawa Timur IXDaerah Pemilihan / Daerah pemilihanuntuk Dewan Perwakilan RakyatRepublik IndonesiaWilayah Daftar Kabupaten : Bojonegoro Tuban ProvinsiJawa TimurPopulasi2.567.521 (2023)[1]Elektorat1.979.375 (2024)[2]Daerah pemilihan saat iniDibentuk2004Kursi11 (2004–09)6 (2009–sekarang)Anggota Ratna Juwita Sari (PKB) Farida Hidayati (PKB) Wihadi Wiyanto (Gerindra) Abidin Fikri (PDI-P) Haeny Relawati Rini Widyastuti (Golkar) Didik Mukria...
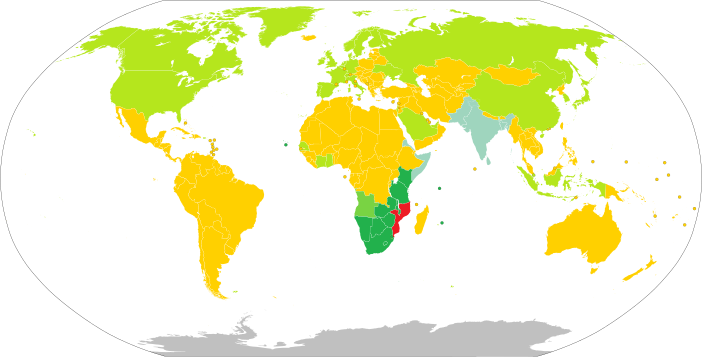
Policy on permits required to enter Mozambique Politics of Mozambique Constitution Human rights Executive President Filipe Nyusi Prime Minister Carlos Agostinho do Rosário Legislature Assembly of the Republic Judiciary Constitutional Council Elections Recent elections General: 201420192024 Political parties Administrative divisions Provinces Districts Postos Foreign relations Ministry of Foreign Affairs Minister: Verónica Macamo Diplomatic missions of / in Mozambique Passport Visa requireme...

Austrian Nordic combined skier David Kreiner Medal record Men's nordic combined Representing Austria Winter Olympics 2010 Vancouver 4 x 5 km team World Championships 2011 Oslo 4 x 5 km team normal hill 2011 Oslo 4 x 5 km team large hill 2001 Lahti 4 x 5 km team 2005 Oberstdorf 4 x 5 km team David Kreiner (born 8 March 1981 in Kitzbühel) is an Austrian Nordic combined skier who has competed since 1998. At the 2010 Winter Olympics, he won gold in the 4 x 5 team event. Kreiner also won tw...

Pour les articles homonymes, voir Érosion (homonymie). Effet de la combinaison de l'érosion éolienne et hydrique (Coyote Buttes, Vermilion Cliffs National Monument, États-Unis). Risque d'érosion des sols (Europe méditerranéenne). faible modérée élevé lacs, mers, océans roche nue zones urbaines absence de donnée en dehors de l'objet de l'étude En géomorphologie, l’érosion est le processus de dégradation et de transformation du relief, et donc des sols, roches, berges et lit...

Namma Metro's Purple Line metro station and upcoming interchange for Orange Line Mysuru Road Namma Metro stationNamma Metro train at Mysuru Road metro station heading towards KengeriGeneral informationLocationMysore Rd, Muthachari Industrial Estate, Deepanjali Nagar, Bengaluru, Karnataka 560039Coordinates12°56′47″N 77°31′47″E / 12.946507°N 77.529780°E / 12.946507; 77.529780Owned byBangalore Metro Rail Corporation Ltd (BMRCL)Operated byNamma MetroLine(s)Purp...