Dalbavancin
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Lushun atau Lüshunkou (bahasa Tionghoa: 旅顺口 ; Hanzi tradisional: 旅順口 ; hanyu pinyin: Lǚshùnkǒu) adalah sebuah kota pelabuhan di Tiongkok, dahulu dikenal dengan nama Port-Arthur (Порт Артур) pada masa pemerintahan Rusia dan Ryojun (旅順) pada masa pemerintahan Jepang. Terletak di ujung Semenanjung Liaodong, kota ini adalah bagian dari Distrik Dalian dan Provinsi Liaoning. Pada tahun 2001 penuduknya mencapai 210.000 jiwa. Lushun Nama Tionghoa Hanzi tradision...

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Cowhill, Gloucestershire – berita · surat kabar · buku · cendekiawan · JSTOR Cowhill adalah sebuah desa di South Gloucestershire, Inggris. Desa ini terletak di selatan Oldbury. Tempat yang paling terkena...

Disambiguazione – Se stai cercando l'omonimo sottotenente medaglia d'oro al valor militare, vedi Paolo Ferrario (sottotenente). Paolo Ferrario Ferrario al Cesena nel 1970 Nazionalità Italia Altezza 178 cm Peso 82 kg Calcio Ruolo Allenatore (ex attaccante) Termine carriera 1975 - giocatore Carriera Giovanili 1950-1959 Milan Squadre di club1 1959-1960 Milan5 (0)1960-1961→ Lazio7 (1)1961 Milan0 (0)1961-1962→ Lazio7 (1)1962-1963→ Simmenthal-Monza27...

العلاقات اللوكسمبورغية المالطية لوكسمبورغ مالطا لوكسمبورغ مالطا تعديل مصدري - تعديل العلاقات اللوكسمبورغية المالطية هي العلاقات الثنائية التي تجمع بين لوكسمبورغ ومالطا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين:...

Social class in the United States The American upper class is a social group within the United States consisting of people who have the highest social rank, due to a lineage associated with wealth, pedigree, and economic wealth.[1][2] The American upper class is distinguished from the rest of the population due to the fact that its primary source of income consists of assets, investments, and capital gains rather than wages and salaries. The American upper class is estimated t...

Norse personification of old age For other uses, see Elli (disambiguation). A depiction of Elli wrestling Thor (1919) by Robert Engels. In Norse mythology (a subset of Germanic mythology), Elli (Old Norse: [ˈelːe], old age[1]) is a personification of old age who, in the Prose Edda book Gylfaginning, defeats Thor in a wrestling match.[2] Gylfaginning In Gylfaginning, Thor and his companions Loki and Þjálfi are in the hall of the jötunn Útgarða-Loki where they mee...

2011 2021 Élections départementales de 2015 dans la Nièvre 34 sièges au sein du conseil départemental les 22 et 29 mars 2015 Type d’élection Élections départementales Campagne Du 9 mars 2015 au 21 mars 2015 Du 23 mars 2015 au 28 mars 2015 Corps électoral et résultats Population 225 952 Inscrits au 1er tour 161 979 Votants au 1er tour 85 847 53,00 % Votes exprimés au 1er tour 81 318 Votes blancs au 1er tour 3 193 Votes nuls au 1er tour ...

County in Florida, United States This article is about the Florida county. For the 2010 novel by John Brandon, see Citrus County (novel). County in FloridaCitrus CountyCountyOld Citrus County Courthouse SealLocation within the U.S. state of FloridaFlorida's location within the U.S.Coordinates: 28°51′N 82°31′W / 28.85°N 82.52°W / 28.85; -82.52Country United StatesState FloridaFoundedJune 2, 1887Named forCitrus trees (previously a major industry in the ...

イスラームにおける結婚(イスラームにおけるけっこん)とは、二者の間で行われる法的な契約である。新郎新婦は自身の自由な意思で結婚に同意する。口頭または紙面での規則に従った拘束的な契約は、イスラームの結婚で不可欠だと考えられており、新郎と新婦の権利と責任の概要を示している[1]。イスラームにおける離婚は様々な形をとることができ、個�...

Argentine diplomat Elena HolmbergBornElena Angélica Dolores Holmberg Lanusse(1931-05-24)24 May 1931Buenos Aires, ArgentinaDisappeared20 December 1978 (aged 47)Buenos Aires, ArgentinaOccupationDiplomat Elena Angélica Dolores Holmberg Lanusse (24 May 1931 – disappeared 20 December 1978), better known as Elena Holmberg, was an Argentine diplomat who was kidnapped and assassinated in 1978. Distinguished for being the first woman to graduate from the Institute of Foreign Services of the N...

Susanna (HWV 66) est un oratorio en trois actes de Georg Friedrich Haendel. Le livret est en anglais et a été attribué sans certitude à Newburgh Hamilton même si on pense aujourd'hui qu'il est plutôt l'œuvre du poète et dramaturge Moses Mendes[1] (mort en 1758). L'intrigue se fonde sur l'histoire de la chaste Suzanne, telle que rapportée dans le chapitre 13 du Livre de Daniel dans l'Ancien Testament. Haendel composa la musique pendant l'été 1748 et la première eut lieu pendant la...

Capital and largest city of Yemen For other uses, see Sanaa (disambiguation). Not to be confused with Sena, Yemen or Saana. City in Capital's secretariat, YemenSanaa صَنْعَاء Ṣanʿāʾ 𐩮𐩬𐩲𐩥CityClockwise from top: Sanaʽa skyline, the Old City, National Museum of Yemen, Gate of Yemen, Al Saleh MosqueNickname: ʾAmānat Al-ʿĀṣimah (أَمَانَة ٱلْعَاصِمَة)Map of the citySanaaShow map of YemenSanaaShow map of West and Central AsiaCoordinates: 15°20...

此條目可参照英語維基百科相應條目来扩充。 (2021年5月6日)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 约翰斯顿环礁Kalama Atoll 美國本土外小島嶼 Johnston Atoll 旗幟颂歌:《星條旗》The Star-Spangled Banner約翰斯頓環礁�...

British illustrator and cartoonist (1820–1914) SirJohn TennielSelf-portrait of John Tenniel, c. 1889Born(1820-02-28)28 February 1820London, EnglandDied25 February 1914(1914-02-25) (aged 93)London, EnglandNationalityBritishKnown forIllustration, children's literature, political cartoons Sir John Tenniel (/ˈtɛniəl/;[1] 28 February 1820 – 25 February 1914)[2] was an English illustrator, graphic humourist and political cartoonist prominent in th...

Airport in Bray-sur-SommeAlbert – Picardie AirportAéroport d'Albert – PicardieIATA: BYFICAO: LFAQSummaryAirport typePublicOperatorSociété d’Exploitation Aéroport Albert Picardie (SEAAP)ServesAlbert, Somme, FranceLocationBray-sur-SommeElevation AMSL363 ft / 111 mCoordinates49°58′12″N 002°41′33″E / 49.97000°N 2.69250°E / 49.97000; 2.69250Websitewww.aeroportalbertpicardie.comMapAlbert – PicardieLocation of airport in FranceRunways Di...

В Википедии есть статьи о других людях с такой фамилией, см. Перминов. Перминов Дмитрий Сергеевич Дата рождения 3 апреля 1979(1979-04-03) (45 лет) Место рождения Омск Род деятельности политик, депутат Государственной думы Российской Федерации Принадлежность СССР → Рос...

Halaman ini berisi artikel tentang sejarah Poso sebelum tahun 1959. Untuk sejarah Poso setelah bergabung dengan Indonesia, lihat Sejarah Kabupaten Poso. Garis waktu Kabupaten Poso Poso di bawah kekuasaan Luwu (1600—1897) Afdeling Poso (1919—1942; 1946—1948) Afdeling Poso (1942—1945) Afdeling Poso (1948—1949) Daerah Otonom Poso (1949—1959) Kabupaten Poso (1959—sekarang) Sejarah Poso (bahasa Inggris: History of Poso) dimulai sejak zaman batu hingga zaman megalitikum. Wilayah i...

A girandole with mirror A girandole (/ˈdʒɪrəndəʊl/) is an ornamental branched candle holder consisting of several lights that may be on a stand or mounted on the wall, either by itself or attached to a mirror.[1][2] Girandole has been used to refer to a number of different objects and designs; the early girandoles were candelabras decorated with crystals looking like a chandelier on a stand, and at one time it was also used to describe all candelabras and chandeliers, wi...
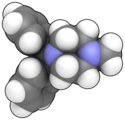
Medication for motion sickness or vertigo CyclizineClinical dataTrade namesMarezine, Valoid, Nausicalm, othersAHFS/Drugs.comConsumer Drug InformationPregnancycategory AU: A Routes ofadministrationBy mouth, IM, IVATC codeR06AE03 (WHO) Legal statusLegal status AU: S3 (Pharmacist only) UK: P (Pharmacy medicines) US: OTC OTC (Netherlands) Pharmacokinetic dataMetabolismN-demethylated to inactive norcyclizine[1]Elimination half-life20 hoursIdentifiers IUPAC nam...

Nakedsingolo discograficoScreenshot tratto dal video del branoArtistaDev FeaturingEnrique Iglesias Pubblicazione20 dicembre 2011 Durata3:58 Album di provenienzaThe Night the Sun Came Up GenereEurodanceElectroDance pop EtichettaUniversal Republic Records ProduttoreThe Cataracs Registrazione2011 FormatiDownload digitale Dev - cronologiaSingolo precedenteSunrise(2011)Singolo successivoTurn the World On(2012) Enrique Iglesias - cronologiaSingolo precedenteI Like How It Feels(2011)Singolo successi...

