Ceftobiprole
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Antonín Panenka Informasi pribadiNama lengkap Antonín PanenkaTanggal lahir 2 Desember 1948 (umur 75)Tempat lahir Praha, CekoslowakiaTinggi 1,78 m (5 ft 10 in)Posisi bermain GelandangKarier junior1958–1967 Bohemians PrahaKarier senior*Tahun Tim Tampil (Gol)1967–1981 Bohemians Praha 230 (76)1981–1985 Rapid Vienna 127 (63)1985–1987 VSE St. Pölten 1987–1989 SK Slovan Wien Total 357 (139)Tim nasional1973–1982 Cekoslowakia 59 (17) * Penampilan dan gol di klub sen...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Januari 2017. artikel ini tidak memiliki pranala ke artikel lain. Tidak ada alasan yang diberikan. Bantu kami untuk mengembangkannya dengan memberikan pranala ke artikel lain secukupnya. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Artikel ini...

本條目存在以下問題,請協助改善本條目或在討論頁針對議題發表看法。 此條目需要补充更多来源。 (2018年3月17日)请协助補充多方面可靠来源以改善这篇条目,无法查证的内容可能會因為异议提出而被移除。致使用者:请搜索一下条目的标题(来源搜索:羅生門 (電影) — 网页、新闻、书籍、学术、图像),以检查网络上是否存在该主题的更多可靠来源(判定指引)。 �...

Julien CarraggiInformasi pribadiKebangsaanBelgiaLahir2 Juli 2000 (umur 23)Brussel, BelgiaTempat tinggalBrussel, BelgiaPeganganKananPelatihWouter ClaesGanda & tunggal putraPeringkat tertinggi70 (MS 30 Mei 2023)583 (MD bersama Jona van Nieuwkerke 18 Oktober 2022)Peringkat saat ini70 (MS), 1240 (MD bersama Elias Bracke) (6 Juni 2023) Rekam medali Bulu tangkis putra Mewakili NOC-Campuran Olimpiade Remaja 2018 Buenos Aires Beregu campuran Profil di BWF Julien Carraggi (lahir 2 Juli 2...

Географическая энциклопедия Украиныукр. Географічна енциклопедія України[1] Язык оригинала украинский[1] Оригинал издан 1989[1], 1990[1] и 1993[1] Географическая энциклопедия Украины (укр. Географічна енциклопедія України) — первое украинское ...

Untuk buah-buahan, lihat Pepaya. Betik Anabas testudineus Ikan betik, Anabas testudineusStatus konservasiRisiko rendahIUCN166543 TaksonomiKerajaanAnimaliaFilumChordataKelasActinopteriOrdoAnabantiformesFamiliAnabantidaeGenusAnabasSpesiesAnabas testudineus Bloch, 1792 lbs Betik,[1] puyo-puyo,[2] puyu,[3] bato,[4] harfan[5] atau betok[6] (Anabas testudineus) adalah nama sejenis ikan air tawar dan payau, seluruh tubuhnya berwarna hitam sampai hijau ...

Cet article est une ébauche concernant une localité luxembourgeoise. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Pulvermühl (lb) Polvermillen (de) Pulvermühle Le quartier. Administration Pays Luxembourg Canton Luxembourg Commune Luxembourg Démographie Population 393 hab.[1] (31 décembre 2023) Densité 1 585 hab./km2 Géographie Coordonnées 49° 36′ 32″ nord, 6° ...

Questa voce sull'argomento calciatori brasiliani è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Jonas Jessue da Silva Júnior Nazionalità Brasile Altezza 181 cm Peso 77 kg Calcio Ruolo Difensore, centrocampista difensivo Squadra Joinville Carriera Giovanili 2003 Mirassol Squadre di club1 2004-2007 São Caetano6 (0)2007-2008 Internacional18 (0)2009→ Sport Recife4 (0)...

МифологияРитуально-мифологическийкомплекс Система ценностей Сакральное Миф Мономиф Теория основного мифа Ритуал Обряд Праздник Жречество Мифологическое сознание Магическое мышление Низшая мифология Модель мира Цикличность Сотворение мира Мировое яйцо Мифическое �...

В Википедии есть статьи о других людях с такой фамилией, см. Тихомиров; Тихомиров, Владимир. Владимир Васильевич Тихомиров Дата рождения 10 декабря 1897(1897-12-10) Место рождения д. Кириллово, Варнавинский уезд, Костромская губерния, Российская империя Дата смерти 30 января 1944(...

Brazilian footballer For other people named Marcos Júnior, see Marcos Júnior (disambiguation). Marcos Júnior Personal informationFull name Marcos Antônio Candido Ferreira JúniorDate of birth (1995-05-13) 13 May 1995 (age 28)Place of birth Rio de Janeiro, BrazilHeight 1.79 m (5 ft 10 in)Position(s) MidfielderTeam informationCurrent team NáuticoYouth career2014–2015 BanguSenior career*Years Team Apps (Gls)2015–2016 Bonsucesso 19 (0)2016 Volta Redonda 15 (4)2017 Am�...

صورة لاحد المصانع التي تستخدم المفعلات الحيوية هندسة العمليات الحيوية هو تخصص في التقانة الحيوية أو الهندسة الحيوية أو الهندسة الكيميائية أو الهندسة الزراعية. تتناول هندسة العمليات الحيوية تصميم وتطوير المعدات والعمليات لتصنيع منتجات مثل الطعام والأعلاف والأدوية والمو...

Der Begriff Technologieknoten (englisch technology node) bezeichnet in der Halbleitertechnik einen Meilenstein für die Definition einer Herstellungsprozessgeneration und bezieht sich im Wesentlichen auf die kleinste fotolithografisch herstellbare Strukturgröße. Seit 1997 wird er durch die International Technology Roadmap for Semiconductors (ITRS) definiert. Der Begriff selbst ist jedoch sehr abstrakt und beschreibt nur grob den technologischen Fortschritt der Branche. So unterscheiden sich...
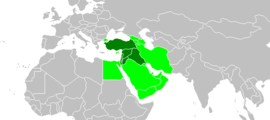
الشرق الأدنىمعلومات عامةجزء من The Near and Middle East (en) القارة آسيا الإحداثيات 32°48′N 35°36′E / 32.8°N 35.6°E / 32.8; 35.6 يدرسه دراسات الشرق الأدنى لديه جزء أو أجزاء تركياسوريالبنان تعديل - تعديل مصدري - تعديل ويكي بيانات الشرق الأدنى الشرق الأدنى أو الشرق القريب مصطلح يستخدمه عل�...

Come OnSampul edisi regulerLagu oleh CN Bluedari album Code Name BlueSisi-BWake UpMy MiracleDirilis1 Agustus 2012(see release history)FormatCD singel, unduhan digitalDirekam2012GenreRockDurasi3:31LabelWarner Music JapanPenciptaJung Yong-hwa, Kenji TamaiProduserLee Jong-hyun Come On adalah lagu dari band rock asal Korea Selatan CNBLUE, ditulis oleh Jung Yong-hwa, diterjemahkan ke dalam bahasa Jepang oleh Kenji Tamai dan disusun oleh Lee Jong-hyun. Singel ini dirilis pada tanggal 1 Agustus 2012...

American baseball player (born 1973) Baseball player Bob HowryHowry with the Chicago Cubs in 2007PitcherBorn: (1973-08-04) August 4, 1973 (age 50)Phoenix, Arizona, U.S.Batted: LeftThrew: RightMLB debutJune 21, 1998, for the Chicago White SoxLast MLB appearanceJuly 28, 2010, for the Chicago CubsMLB statisticsWin–loss record45–52Earned run average3.84Strikeouts653 Teams Chicago White Sox (1998–2002) Boston Red Sox (2002–2003) Cleveland Indians (2004–...

Bayu Jagat Kasiops Kasrem 151/Binaiya Informasi pribadiLahir19 Agustus 1974 (umur 49)Palembang, Sumatera SelatanAlma materAkademi Militer (1997)Karier militerPihak IndonesiaDinas/cabang TNI Angkatan DaratMasa dinas1997—sekarangPangkat KolonelNRP11970032370874SatuanInfanteriSunting kotak info • L • B Kolonel Inf. Bayu Jagat, S.I.P., M.H. (lahir Agustus 1974) adalah seorang perwira menengah TNI-AD yang saat ini menjabat sebagai Kasiops Kasrem 151/Binaiya.[1 ...

Nazi plan to create a resistance force which would operate behind enemy linesvteGerman–Polish WarsHoly Roman Empire 963 967 972 979 1003-1018 1028-1031 1074 1109 1146 1157 1184 1278 Brandenburg 1247-1252 1265-1278 1269-1272 1296 1311-1312 1316 1326-1329 1370 1476-1482 1656-1657 Teutonic Order 1308 1326–1332 1409–1411 1414 1419 1422 1431–1435 1454–1466 1467–1479 1519–1521 Prussia 1733-1735 1792-1797 1795 1806-1807 1813-1814 1846 1848 1914-1918 Weimar Republic 1918-1919 1919 1919-...

This article is about the AM radio station in Kilgore, Texas. For the FM station in Tyler, Texas formerly known as KDOK, see KRWR. For the co-owned station in Tyler, Texas formerly known as KDOK, see KYZS. Radio station in Kilgore, TexasKDOKKilgore, TexasBroadcast areaLongview-Marshall areaFrequency1240 kHzBrandingAll Hit Radio K-DOKProgrammingFormatClassic hitsAffiliationsKilgore BulldogsOwnershipOwnerChuck Conrad(Chalk Hill Communications, LLC)Sister stationsKZQXKYZSHistoryFirst air date193...

Pour les articles homonymes, voir Ormoc. OrmocNom officiel (en) City of OrmocGéographiePays PhilippinesRégion Visayas orientalesProvince LeyteSuperficie 613,6 km2Altitude 234 mCoordonnées 11° 00′ 38″ N, 124° 36′ 27″ EDémographiePopulation 230 998 hab. (2020)Densité 376,5 hab./km2 (2020)FonctionnementStatut Ville-composante indépendante (en)Chef de l'exécutif Lucy Torres (en) (depuis le 30 juin 2022)Dépenses 1,6 G₱ (202...



