Tetronska kiselina je hemijsko jedinjenje koje se klasifikuje kao γ-lakton i ima molekularnu formulu C4H4O3.
Ona se interkonvertuje između keto i enol tautomera:[4]
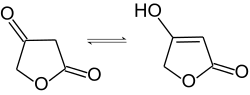
Mnogi prirodni proizvodi kao što je askorbinska kiselina (vitamin C), penicilinska kiselina, pulvinske kiseline, i abisomicini poseduju β-keto-γ-butirolaktonski motiv tetronske kiseline.[5]
U organskoj sintezi, ona se koristi kao prekurzor za druge substituisane i kondenzovane furane i butenolida.[6][7] Ona isto tako formira strukturnu osnovu klase pesticida, poznate kao tetronsko kiselinski insekticidi, koja obuhvata spirodiklofen i spiromesifen.[8]
Vidi još
Reference
- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003. уреди
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ „2,4(3H,5H)-Furandione”. Sigma-Aldrich.
- ^ Abdou, Moaz M.; El-Saeed, Rasha A.; Abozeid, Mohamed A.; Elattar, Khaled M.; Zaki, E.G.; Barakat, Y.; Ibrahim, V.; Fathy, Mahmoud; Amine, M.; Bondock, Samir (2015). „Advancements in tetronic acid chemistry. Part 1: Synthesis and reactions”. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2015.11.004.
- ^ Georgiadis, Dimitris; Zografos, Alexandros (2006). „Synthetic Strategies towards Naturally Occurring Tetronic Acids”. Synthesis. 2006 (19): 3157. doi:10.1055/s-2006-950202.
- ^ „Tetronic acid”. Alfa Aesar.
- ^ Schmidt, Diane Grob; Seemuth, Paul D.; Zimmer, Hans (1983). „Substituted .gamma.-butyrolactones. Part 31. 2,4(3H,5H)-Furandione: Heteroannulations with aromatic o-amino carbonyl compounds and condensations with some vic-polyones”. The Journal of Organic Chemistry. 48 (11): 1914. doi:10.1021/jo00159a029.
- ^ „Classification of Insecticides”. alanwood.net.