|
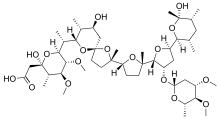
Maduramicin
Maduramicin
|
|
|
| Klinički podaci
|
| AHFS/Drugs.com
|
Monografija
|
| Identifikatori
|
| CAS broj
|
79356-08-4
|
| ATCvet kod
|
QP51AX10
|
| PubChem[1][2]
|
68595
|
| ChemSpider[3]
|
61862
|
| UNII
|
5U912U22T2  Y Y
|
| ChEMBL[4]
|
CHEMBL1909066  Y Y
|
| Hemijski podaci
|
| Formula
|
C47H80O17
|
| Mol. masa
|
917,128
|
| SMILES
|
eMolekuli & PubHem
|
| InChI |
|---|
InChI=1S/C47H80O17/c1-23-18-24(2)45(9,51)61-36(23)31-19-32(58-35-20-30(53-10)40(55-12)28(6)57-35)42(59-31)44(8)15-14-33(60-44)43(7)16-17-46(64-43)21-29(48)25(3)37(62-46)26(4)38-41(56-13)39(54-11)27(5)47(52,63-38)22-34(49)50/h23-33,35-42,48,51-52H,14-22H2,1-13H3,(H,49,50)/t23-,24+,25+,26+,27-,28-,29-,30-,31+,32-,33+,35+,36-,37-,38-,39-,40-,41-,42+,43-,44-,45-,46+,47+/m0/s1
Key: RWVUEZAROXKXRT-VQLSFVLHSA-N  Y Y |
|
| Sinonimi
|
Maduramycin
|
| Farmakoinformacioni podaci
|
| Trudnoća
|
?
|
| Pravni status
|
|
Maduramicin je organsko jedinjenje, koje sadrži 47 atoma ugljenika i ima molekulsku masu od 917,128 Da.
Osobine
Reference
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ Ghose, A.K., Viswanadhan V.N., and Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A 102: 3762-3772. DOI:10.1021/jp980230o.
- ↑ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE. (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488-1493. DOI:10.1021/ci000392t. PMID 11749573.
- ↑ Ertl P., Rohde B., Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714-3717. DOI:10.1021/jm000942e. PMID 11020286.
Literatura
Vanjske veze
|
|