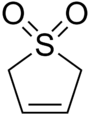
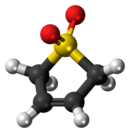
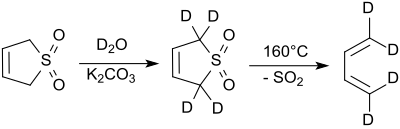
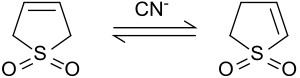
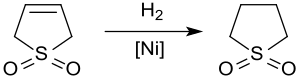
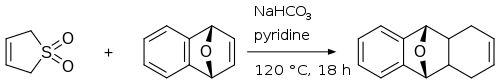
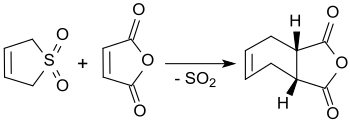
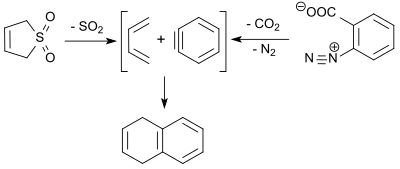
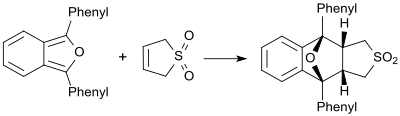
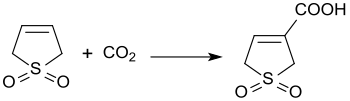
Sulfolene
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Aero-Tropics Air Services IATA ICAO Kode panggil HC ATI Aerotropics Didirikan1995PenghubungPulau HornBandar Udara Internasional CairnsKota fokusPulau HornBandar Udara Internasional CairnsPulau CoconutArmada14 (Mei 2006)Tujuan11 (2 negara)Perusahaan indukKeluarga LippmannKantor pusatPulau Horn, Queensland, AustraliaSitus webhttp://www.aero-tropics.com.au/ Aero-Tropics Air Services merupakan sebuah maskapai penerbangan yang berbasis di Cairns, Queensland, Australia. Maskapai ini mengoperasikan ...

Konten dan perspektif penulisan artikel ini hanya berpusat pada sudut pandang dari negara Indonesia dan tidak menggambarkan wawasan global pada subjeknya. Silakan bantu mengembangkan atau bicarakan artikel ini di halaman pembicaraannya, atau buat artikel baru, bila perlu. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Penduduk yang berkumpul di rumah Ketua Tonarigumi di Jepang Tonarigumi (Jepang: 隣組) atau dalam Bahasa Indonesia artinya kerukunan tetangga (sekarang...

Shopping mall in Ontario, CanadaEglinton Square Shopping CentreEglinton Square in 2023LocationToronto, Ontario, CanadaCoordinates43°43′25″N 79°17′59″W / 43.7236°N 79.2996°W / 43.7236; -79.2996Address1 Eglinton SquareOpening date1953DeveloperOxford PropertiesOwnerKingsett Capital, Bentall Kennedy (Canada) LPNo. of stores and services80+No. of anchor tenants2Total retail floor area279,000 sq ft (25,900 m2)No. of floors1Websiteeglintonsquare.ca ...

Railway station in Cumbria, England For the station in Ohio, United States, see Green Road station. Green RoadGeneral informationLocationMillom Without, CopelandEnglandCoordinates54°14′40″N 3°14′43″W / 54.2444234°N 3.2453885°W / 54.2444234; -3.2453885Grid referenceSD189839Owned byNetwork RailManaged byNorthern TrainsPlatforms2Tracks2Other informationStation codeGNRClassificationDfT category F2HistoryOriginal companyWhitehaven and Furness Junction RailwayPre...

Шалфей обыкновенный Научная классификация Домен:ЭукариотыЦарство:РастенияКлада:Цветковые растенияКлада:ЭвдикотыКлада:СуперастеридыКлада:АстеридыКлада:ЛамиидыПорядок:ЯсноткоцветныеСемейство:ЯснотковыеРод:ШалфейВид:Шалфей обыкновенный Международное научное наз...

Village in Devon, England Not to be confused with Morley. This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Moreleigh – news · newspapers · books · scholar · JSTOR (February 2013) (Learn how and when to remove this message) Human settlement in EnglandMoreleighAll Saints ChurchMoreleighLocation within DevonOS&#...

Teologi (bahasa Yunani θεος, theos, ], Tuhan, dan λογια, logia, kata-kata, ucapan, atau wacana) atau kadang disebut ilmu agama adalah wacana yang berdasarkan nalar mengenai agama, spiritualitas dan Tuhan. Dengan demikian, teologi adalah ilmu yang mempelajari segala sesuatu yang berkaitan dengan keyakinan beragama atau ilmu tentang Tuhan. Teologi meliputi segala sesuatu yang berhubungan dengan Tuhan. Istilah teologisasi merujuk pada kecenderungan untuk menggunakan sudut pandang teolog...

Азиатский барсук Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:СинапсидыКласс:Мле�...

此條目可参照英語維基百科相應條目来扩充。 (2017年8月)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 密西西比州 美國联邦州State of Mississippi 州旗州徽綽號:木蘭之州地图中高亮部分为密西西比州坐标:30°13'N�...

British racing driver (born 1940) Richard AttwoodRichard Attwood at the 1968 German Grand Prix.Born (1940-04-04) 4 April 1940 (age 84)Wolverhampton, Staffordshire, EnglandFormula One World Championship careerNationality BritishActive years1964–1965, 1967–1969TeamsBRMReg Parnell RacingCooperLotusEntries17 (16 starts)Championships0Wins0Podiums1Career points11Pole positions0Fastest laps1First entry1964 British Grand PrixLast entry1969 Monaco Grand Prix Richard James David Dickie At...

Ця стаття потребує додаткових посилань на джерела для поліпшення її перевірності. Будь ласка, допоможіть удосконалити цю статтю, додавши посилання на надійні (авторитетні) джерела. Зверніться на сторінку обговорення за поясненнями та допоможіть виправити недоліки. Мат...

Building in New York City, New YorkEast CampusEast Campus' western facadeGeneral informationAddress70 Morningside Drive, New York City, New YorkOpened1981OwnerColumbia UniversityTechnical detailsFloor count20Design and constructionArchitect(s)Gwathmey Siegel & Associates Architects East Campus is a prominent building on the Morningside Heights campus of Columbia University in New York City, located along Morningside Drive between 117th and 118th Streets. One of the tallest buildings in th...
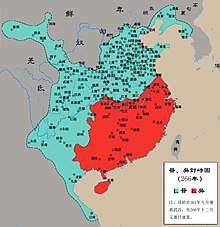
Pour un article plus général, voir Trois Royaumes de Chine. Pour les articles homonymes, voir Wu. Royaume de Wu(zh) 吳 (Wú) 229–280 Territoires des Trois Royaumes de Chine en 262.Le royaume de Wu est représenté en vert.Informations générales Statut Monarchie Capitales Wuchang (222 – 229, 265 – 266)Jianye (229 – 265, 266 – 280) Langue(s) Chinois Religion Bouddhisme, taoïsme, confucianisme, religion traditionnelle chinoise Monnaie Anciennes monnaies chinoises Démograph...

Cognitive process to choose a course of action or belief This article is about decision-making as analyzed in psychology. For a broader discipline, see Decision theory. For decision-making in groups, see Group decision-making. Sample flowchart representing a decision process when confronted with a lamp that fails to light In psychology, decision-making (also spelled decision making and decisionmaking) is regarded as the cognitive process resulting in the selection of a belief or a course of a...

57°02′06″N 2°18′25″W / 57.035°N 2.307°W / 57.035; -2.307 Map of Scotland showing the present-day committee area of Kincardine and Mearns Kincardine and Mearns is one of six area committees of the Aberdeenshire council area in Scotland. It has a population of 38,506 (2001 Census). There are significant natural features in this district including rivers, forests, mountains and bogs (known locally as mosses). Transport links with Aberdeen have encouraged rapid...

لاعب كرة قدم تسمية الإناث لاعبة كرة قدم فرع من لاعب منافسة [لغات أخرى] المجال كرة القدم، وكرة القدم للرجال، وكرة القدم للسيدات، وكرة القدم البارالمبية، وكرة القدم للمكفوفين، وكرة القدم المصغرة تعديل مصدري - تعديل لاعب كرة القدم ه...

Yosua 9Kitab Yosua lengkap pada Kodeks Leningrad, dibuat tahun 1008.KitabKitab YosuaKategoriNevi'imBagian Alkitab KristenPerjanjian LamaUrutan dalamKitab Kristen6← pasal 8 pasal 10 → Yosua 9 (disingkat Yos 9) adalah pasal kesembilan Kitab Yosua dalam Alkitab Ibrani dan Perjanjian Lama di Alkitab Kristen yang memuat riwayat Yosua dalam memimpin orang Israel menduduki tanah Kanaan.[1] Pasal ini berisi riwayat akal orang Gibeon supaya tidak dimusnahkan oleh orang Israel.[...

Pour les articles homonymes, voir Guéret (homonymie). Guéret De haut en bas et de gauche à droite : office de tourisme ; palais de justice ; église Saint-Pierre-et-Saint-Paul ; présidial ; fontaine des Trois-Grâces ; musée d'Art et d'Archéologie ; théâtre à l'italienne. Blason Logo Administration Pays France Région Nouvelle-Aquitaine Département Creuse (préfecture) Arrondissement Guéret(chef-lieu) Intercommunalité Communauté d'agglomératio...

مطبخ أمريكيمعلومات عامةصنف فرعي من مطبخ أمريكا الشمالية جزء من ثقافة الولايات المتحدة الثقافة التواصل عبر الثقافات أصيل في الولايات المتحدة تعديل - تعديل مصدري - تعديل ويكي بيانات سلطعون أزرق حساء البطلينوس، إنجلترا الجديدة المطبخ الأمريكي، هي الأكلات التي اخذتها الولاي...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Mann Gulch fire – news · newspapers · books · scholar · JSTOR (December 2020) (Learn how and when to remove this message)United States historic placeMann Gulch Wildfire Historic DistrictU.S. National Register of Historic PlacesU.S. Historic district Investigat...