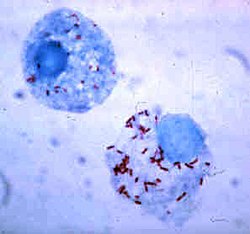
Rickettsia rickettsii
Rickettsia rickettsii is a Gram-negative, intracellular, cocco-bacillus bacterium that was first discovered in 1896.[1] Having a reduced genome, the bacterium harvests nutrients from its host cell to carry out respiration, making it an organo-heterotroph. Maintenance of its genome is carried out through vertical gene transfer where specialization of the bacterium allows it to shuttle host sugars directly into its TCA cycle.[2] Other characteristics of the bacteria include membrane proteins that are useful in the identification of R. rickettsii strains and useful in targeting from antibiotics. A capsule encircling the bacterium allows for attachment to host cells and additionally acts as a defense mechanism for resisting phagocytosis. Varying strains of R. rickettsii have different genotypes and phenotypes that alter the pathogenicity, virulence, and the appearance of the bacteria.[3][4][5] R. rickettsii is the causative agent of Rocky Mountain Spotted Fever and is transferred to its host via a tick bite. It is one of the most pathogenic Rickettsia species[6] and affects a large majority of the Western Hemisphere, most commonly the Americas.[7] The pathogenic agent has been found on every continent, except Antarctica; however, Rocky Mountain Spotted Fever occurs mostly in North, Central, and South America.[7] This prevalence is due to R. rickettsii ability to thrive in warm, damp environments.[8] These environments provide sufficient conditions for the amplification of the bacteria within a vertebrate host, such as a horse or dog. The bacteria are transmitted through a vector, such as a tick, to a vertebrate host where it can then be amplified and passed on to a person, resulting in the zoonotic disease.[9] Headache, high fever, and spotted rash are some effects of the disease with more severe cases resulting in organ damage and coma.[10][11][12] Antibiotics, such as doxycycline, target the ribosome of R. rickettsii in order to inhibit protein synthesis of the bacteria, providing a form of treatment for the disease.[13] PhysiologyMetabolic pathwaysR. rickettsii are obligate intracellular bacteria, meaning they need a host cell in order to replicate and survive. In fact, no glycolytic enzymes for the breakdown of intact glucose remain in R. rickettsii's genome.[14] It is theorized that while R. rickettsii once possessed complete, complex metabolic pathways that allowed it to survive outside a host, evolutionary pressures caused progressive genomic reduction [15] that now limits metabolism to the tricarboxylic acid cycle (TCA).[14] Energy is primarily obtained through a combination of oxidative phosphorylation of imported host carbon sources and direct harvesting of ATP via a ATP/ADP transmembrane pump.[16] Remnants of these lost metabolic pathways can be seen in analysis of R. rickettsii's genome, which contain some identified remnant enzymes of pathways that remain unfunctional in vivo.[15] This decrease in available metabolic pathways has left R. rickettsii largely dependent on a range of transport systems to harvest essential amino acids, nucleic acids, and other metabolites from its host. The primary carbon source of R. rickettsii is pyruvate, though many other amino acids and TCA cycle intermediates such as glutamine, glutamate, and malate can be used.[16] For lipid metabolism, a complete map of fatty acid synthesis enzymes have been found, allowing R. rickettsii to construct a viable cell membrane made up of lipopolysaccharides (LPS). Regarding synthesis of a peptidoglycan layer, there is speculation as to whether key components are synthesized internally or imported and modified for use.[14] Pathways for producing critical small molecules such as riboflavin (B2), nicotinamide (B3), pantothenate (B5), pyridoxine (B6), and biotin (B7) are all missing key enzymes, forcing R. rickettsii to rely solely on transmembrane transport proteins.[14][16] Overall, R. rickettsii has a genome that does not encode many of the enzymes and proteins that are required for several pathways besides the TCA cycle. These bacteria import many of the intermediates, cofactors, and byproducts from the host cells' metabolic pathways to use for their own synthesis of necessary structures and energy for survival.[2] MorphologyR. rickettsii has many vital proteins within its cellular membranes. One of these proteins is YbgF, which maintains the structure of the cellular membrane. YbgF is found within both the inner and outer membranes along with another protein called TolC. TolC is a transport protein that connects to other transport proteins within the periplasmic space and inner membrane. These two proteins are believed to be associated with pathogenicity of this microbe and serve as specific points that antibodies can bind to in order to prevent the bacteria from interacting with host cells.[3] R. rickettsii has an outer layer or a "microcapsule", in addition to the traditional peptidoglycan cell wall. The function of the microcapsule resembles that of slime layers, or S-layers, of other bacteria. This slime layer consists mostly of polysaccharides and is constantly undergoing changes in reaction to chemical or physiological events. Research to precisely determine the function of the slime layer is currently limited due to high risk of infection while working with this bacterium; however, scientists can infer based on conclusions from other studies that it is likely that this slime layer is used for antiphagocytic properties. This prevents phagocytes from engulfing and killing the R. rickettsii bacteria and allows for attachment to host cells in preparation to penetrate and infect those cells.[17] Pathophysiology While humans are hosts for R. rickettsii, they do not contribute to rickettsial transmission. Rather, the pathogen is maintained through its vector: ticks. R. rickettsii invades vascular endothelial cells that line both small- and medium-sized blood vessels in the host's body.[18] There is an extensive immune response to this pathogen that triggers different pathways in the macrovascular and microvascular systems.[18] As the cells are damaged there is an increased permeability of the vessels (extravasation), microvascular hemorrhages, and necrosis. Additionally, there is evidence that suggests that R. rickettsii possesses the ability to cross the blood-brain barrier into the host's central nervous system, allowing the pathogen to target the brain's vasculature.[18] The pathogen causes changes in the host cell's cytoskeleton that induce phagocytosis.[citation needed] Consequently, R. rickettsii replicates further and infects other cells in the host's body. R. rickettsii's survival in the immune system cells increases the pathogen's virulence in mammalian hosts.[citation needed] Nitric oxide has the ability to inhibit the pathogen by negatively effecting attachment, intracellular growth, and subversion of the host cell.[19] It does so by depleting R. rickettsii of ATP as nitric oxide targets cytochrome bo oxidase and cytochrome bd oxidase complexes which are necessary for ATP-synthase. The impact of nitric oxide on the metabolism however is significant enough to limit the pathogens ability to attach to host cells. As translation is also majorly ATP-dependent, the introduction of nitric oxide can greatly reduce the process of translation and therefore protein synthesis, making R. rickettsii incapable of subverting the host cell.[19] Understanding of these inhibitory processes may allow for more advance care and possible antimicrobial treatments to be developed. Actin-Based Motility (ABM) is a virulence factor that allows for the pathogen to evade the host's immune cells and spread to neighboring cells. It is suggested that the Sca2 gene, which is an actin-polymerizing determinant, is a distinguishing factor for the Rickettsia family, as R. rickettsii mutants with a Sca2 transposon the bacteria can avoid autophagic processes by host phagocytic cells. This leads to an increase in disease manifestation for the host. R. rickettsii is also able to suppress immune responses while dwelling in infected cells by creating inhibitory proteins such as Rickettsial ankyrin repeat protein 2 (RARP2). RARP2 mediates the fragmentation of TGN, or the trans-Golgi network, causing attenuation of vesicular transport and glycosylation defects in infected host cells. There are two important proteins within the host cell that are affected by these glycosylation defects: TGN46 and major histocompatibility complex class 1 (MHC-I). MHC-I is an important protein for defending against pathogens as it functions as an antigen presenting complex signaling its infection status to lyphocytes. However, since RARP2 causes attenuation of vesicular transport, MHC-I is unable to be transported to the plasma membrane and the infected cell will not be able to alert host immune cells. Thus, the bacterial cells are able to avoid certain immune responses and allow for proliferation within a host cell. This pathogen also has the unique ability, as seen in studies on ticks, to prevent apoptosis. It does so by affecting apoptosis regulators such as caspases, Bcl-2 proteins, or possibly the p53 tumor suppressor pathway. This provides an advantage, allowing R. rickettsii to proliferate further due to prolonged survival and increased time for replication. By doing so, R. rickettsii also increases its transmission abilities. Without this process in place, the pathogen may not survive long enough in the vector to properly replicate and infect other hosts.[20] R. rickettsii is an obligate intracellular alpha proteobacterium that belongs to the Rickettsiacaea family. Within the Rickettsia species, these bacteria are divided into four clades. The clades include the ancestral group, spotted fever group (SFG), typhus group, and transitional group, and the determining factors for classifying into each group depends on phenotypic characteristics, phylogenetic organization, or the type of vector host they inhabit. R. rickettsii falls into the largest group of them all, the SFG group.[21] R. rickettsii has a genome that consists of about 1.27 Mbp with ~1,350 predicted genes, which is smaller compared to most other bacteria. This small genome size allows the bacteria to maintain an intracellular lifestyle with increased pathogenicity from gene reduction. It is maintained in its tick host by transovarial transmission. The multiplication of R. rickettsii is by binary fission inside the cytosol. Genome and phenotypesR. rickettsii is an obligate intracellular alpha proteobacterium that belongs to the Rickettsiaceae family.[6] Within the Rickettsia species, these bacteria are divided into four clades. The clades include ancestral group, spotted fever group (SFG), typhus group, and transitional group. Factors for classifying each group depends on phenotypic characteristics, phylogenetic organization, or the type of vector host they inhabit. R. rickettsii falls into the largest group of them all, the SFG group.[22] It has a genome that consists of about 1.27 Mbp with ~1,350 predicted genes,[4] which is smaller compared to most other bacteria. This small genome size allows the bacteria to maintain an intracellular lifestyle with increased pathogenicity from gene reduction.[23] It is maintained in its tick host by transovarial transmission.[6] The multiplication of R. rickettsii is by binary fission inside the cytosol.[1] Genomic comparison of strainsR. rickettsii has a relatively small genome; however, variances in gene expression between different strains can lead to various functions of the bacteria. For instance, there are two major strains of R. rickettsii called the Iowa and the Sheila Smith strains. The Sheila Smith strain is a virulent strain, while the Iowa strain is an avirulent strain. Microarrays revealed that there were only four distinct differences in the gene expression of R. rickettsii; however, these four changes lead to complete differences in virulence, and thus the niche of the bacteria.[24] A key feature allowing for differentiation is the rickettsial outer membrane protein, rOmpA and rOmpB [4] which contributes to the identification of R. rickettsii strains as virulent. The detection of single nucleotide polymorphisms (SNPs) are used to differentiate these strains.[4] Transmission cycleEcology The most common hosts for R. rickettsii are ticks.[8] Ticks that carry R. rickettsii fall into the family of Ixodidae ticks, also known as "hard-bodied" ticks.[26] Ticks are vectors, reservoirs, and amplifiers of these bacteria.[8] There are currently three known tick species that commonly carry R. rickettsii. The American dog tick (Dermacentor variabilis), mainly found in the eastern United States, is the most common vector for R. rickettsii. The Rocky Mountain wood tick (Dermacentor andersoni), found in the Rocky Mountain States, and the brown dog tick (Rhipicephalus sanguineus), found in select areas of the southern United States, are also known vectors of the pathogen.[26][27] Infecting horses and capybaras, the cayenne tick (Amblyomma cajennense) turns vertebrate hosts into amplifier hosts of the bacteria. Other small rodent species serve as amplifier hosts in the United States and South America.[9] Transmission in ticksTicks can contract R. rickettsii by many means. An uninfected tick can become infected through feeding on the blood of an infected vertebrate host during the larval or nymph stages. This mode of transmission is called transstadial transmission.[28] Once a tick becomes infected with this pathogen, they are infected for life. The pathogen, however, does not harm the tick itself and only causes symptoms in mammals infected by the tick.[29] Both the American Dog Tick and the Rocky Mountain Wood Tick serve as long-term reservoirs for Rickettsia rickettsii, infecting the posterior diverticula of the midgut, the small intestine, and the ovaries.[28] In addition, an infected male tick can transmit the organism to an uninfected female during mating,[30] and infected female ticks can transmit the infection to their offspring in a process known as transovarian passage.[31][32] This process, however, is unlikely to play a major role in the maintenance of R. rickettsii within a population.[33] Notably, R. rickettsii is inefficient at infecting the ovaries of adult female ticks, resulting in a lowered rate of vertical transmission.[33] Rickettsial colonization of the ovaries sees higher success when ticks obtain the pathogen as a larva or nymph.[34] Reduced fecundity is also observed in ticks infected with R. rickettsii.[30] As a result of these limitations, long-term maintenance of R. rickettsii in populations of ticks relies mainly on horizontal transmission through the exchange of bacteria during feedings of infected hosts.[30][35] The duration of tick attachment, bacterial loads in tick saliva, and the transmission efficiency of Rickettsia are important factors underlying transmission from ticks to humans.[36] Transmission in mammalsDue to its confinement in the midgut and small intestine, Rickettsia rickettsii can be transmitted to mammals, including humans. Transmission can occur in multiple ways. The most common way of contraction is by the bite of an infected tick.[32] After getting bitten by an infected tick, R. rickettsiae is transmitted into the bloodstream by tick salivary secretions.[37] The saliva of ticks contain immunomodulatory agents which effect immune defenses in the host. This allows for R. rickettsiae to be transmitted with little to no resistance from the host's immune system.[37]Another way to contract the infection is through contact with an infected host's feces. If an infected host's feces come into contact with an open skin barrier, it is possible for the disease to be transmitted. An uninfected host can become infected when eating food that contains the feces of the infected vector.[37] Having multiple modes of transmission ensures the persistence of R. rickettsii in a population. Additionally, having multiple modes of transmission helps the disease adapt better to new environments and prevents it from becoming eradicated. R. rickettsii has evolved a number of strategic mechanisms, or virulence factors, that allow it to invade the host immune system and successfully infect its host.[38] Clinical manifestationsThe Centers for Disease Control and Prevention states that the diagnosis of Rocky Mountain Spotted Fever (RMSF) must be made based on the clinical signs and symptoms of the patient and then later be confirmed using specialized laboratory tests. However, the diagnosis of Rocky Mountain Spotted Fever is often misdiagnosed due to its non-specific onset. The majority of infections from R. rickettsii occur during the warmer months between April and September due to its most common method of transmission being via tick bite. Symptoms can take 1–2 days to 2 weeks to present themselves within the host.[10] The diagnosis of RMSF is easier when there is a known history of a tick bite or if the rash is already apparent in the affected individual.[39] If not treated properly, the illness may become serious, leading to hospitalization and possible fatality.[40] Signs and symptomsDuring the initial stages of the disease, the infected person may experience headaches, muscle aches, chills, and high fever. Other early symptoms may include nausea, vomiting, loss of appetite, and conjunctival injection (red eyes). Most people infected by R. rickettsii develop a spotted rash, that begins to appear 2 to 4 days after the individual develops a fever. If left untreated, more severe symptoms may develop; these symptoms may include insomnia, compromised mental ability, coma, and damage to the heart, kidneys, liver, lungs, or additional organs.[11][10]  The classic Rocky Mountain Spotted Fever rash occurs in about 90% of patients and develops 2 to 5 days after the onset of fever. The rash can differ greatly in appearance along the progress of the R. rickettsii infection.[11] It is not itchy and starts out as flat pink macules located on the affected individual's hands, feet, arms, and legs.[39] During the course of the disease, the rash may form petechiae and take on a more darkened reddish purple spotted appearance, signifying severe disease.[41] In rarer cases, patients may present with chest pain due to myocarditis. Additionally, rare symptoms include vision impairment and arthritis that may exist as chronic sequelae, lasting anywhere from 10 days to 4 years. Other chronic sequelae include some cases of neurological challenges, such as impaired speech, dysphagia, ataxia, memory loss, cortical blindness, and decreased attention span. Necrosis of skin is another rare case of sequelae.[27][42] Severe infectionsPatients with severe infections may require hospitalization. The more severe symptoms occur later in response to thrombosis (blood clotting) caused by R. rickettsii targeting endothelial cells in vascular tissue.[10][43] One manifestation of this damage is the development of a petechial rash, which in 60% of cases presents within 6 days of initial symptoms. Petechial rashes are indicative of more severe disease progression.[44] Patients may become hyponatremic, experience elevated liver enzymes, and other more pronounced symptoms. It is not uncommon for severe cases to involve respiratory system, central nervous system, gastrointestinal system, or renal system complications. In the case of meningoencephalitis, R. rickettsii causes cellular damage to brain tissue, resulting in inflammation. Additionally, acute respiratory distress syndrome and coagulopathy occur in cases that advance to severe stages of RMSF.[12] This disease is worst for elderly patients, males, African Americans, alcoholics, and patients with G6PD deficiency. The mortality rate for RMSF is 3 to 5 percent in treated cases, but 13 to 25 in untreated cases.[39] Deaths are usually caused by heart and kidney failure.[31] TreatmentRMSF symptoms can vary from moderate to severe cases, and a delay in treatment is often associated with a higher case-fatality rate.[45] The most common and effective treatment for Rocky mountain spotted fever is the anti-microbial agent doxycycline.[46] This antibiotic acts as a bacteriostatic drug by inhibiting protein synthesis via blockage of the 30S ribosomal subunit.[13] Other treatments with chloramphenicol, fluoroquinolones, and macrolides have been explored. However, treatment with only chloramphenicol compared to other treatments (tetracycline-class drugs only, both chloramphenicol and tetracycline-class drugs, and neither drug) was associated with a case-fatality rate three times higher.[45] Chloramphenicol, like doxycycline, also functions as a bacteriostatic drug, but it binds to the 50S ribosomal subunit in order to prevent protein synthesis. Macrolides target the 50S subunit as well; however, they block the exit site for peptides, while chloramphenicol blocks the aminoacyl-tRNA attachment site for transfer RNA.[47] Prevention Prevention of Rocky Mountain Spotted Fever begins with identifying and avoiding vectors that cause exposure including ticks, lice, mites, and fleas. Knowledge of where endemic areas are and taking precautions when inhabiting or traveling may also decrease the likelihood of contracting the disease. There are no vaccines to prevent RMSF. Due to increased antibiotic resistance preemptive antibiotic prophylaxis is strongly discouraged in the United States.[48] When coming into contact with vectors, specifically ticks, there are additional preventative measures that can be taken. Many ticks are present in dense bush, grass, and wooded areas. Ticks also can travel on animals, so caution should be taken with pets. They are under the arms, within and around the ears, inside the belly button, back of the knees, within and around the hair, in between the legs, and around the waist. Clothing can be placed it in the dryer on high for at least 10 minutes. Clothes can washed before drying with hot water. Showering two hours after leaving the outside environment lowers the risk of obtaining Lyme disease, a disease whose vector is ticks, therefore it may assist in reducing the risk of other tick-borne diseases.[49] An alternative or additive measure is the use of Environmental Protection Agency (EPA)-registered insect repellents while in areas prone to ticks. These repellents contain at least one of the following active ingredients; picaridin, IR3535, DEET, Oil of lemon eucalyptus (OLE), para-menthane-doil (PMD), or 2-undecanone. Use of repellent that contains OLE or PMD in infants and toddlers, or until they reach 3 years of age is contraindicated.[49] HistoryThe first documented case of Rocky Mountain Spotted Fever (RMSF) presented in the Boise, Idaho in 1896 after being recognized by Major Marshall H. Wood.[1] At the time of discovery, not much information was known about the disease. It was originally called "Black Measles" due to the infected area turning black during the late stages of the disease.[50] The first clinical description of Rocky Mountain Spotted Fever was reported in Snake River Valley in 1899 by Edward E. Maxey.[51] At the time, 69% of individuals diagnosed with RMSF died.[1] It was theorized that the wood tick played a critical role as a vector of disease, based on apparent correlation between tick exposure and disease, as well as the geographic and seasonal presentation.[52] Howard Ricketts (1871–1910), an associate professor of pathology at the University of Chicago in 1902, was the first to identify and study R. rickettsii on a microbial level.[1] His research consisted of interviewing victims of the disease, as well as collecting infected animals to study. He was also known to inject himself with pathogens to more accurately document their effects. Ricketts was the first to identify the pathogen responsible for RMSF as a gram negative bacillus, and confirm a route of transmission from infected ticks in a guinea pig model.[52] His research provided valuable information on the organism's vector and route of transmission.[1] Simeon Burt Wolbach is credited for the first detailed, published description of the pathogenic agent that causes R. rickettsii in 1919. Wolbach described RMSF using the process of Giemsa staining,[1] and positively identified the bacterium as frequently residing within endothelial cells.[53][52] The once lethal infection RMSF has become curable due to the modern availability of antibiotics. Broad spectrum antibiotics chloramphenicol and tetracycline-class drugs, like doxycycline, were first harnessed as treatment for RMSF in the late 1940s.[52] Before their discovery, 1 in 5 infected patients died.[54] Treatment recommendations changed in the 1990s to support primary therapeutic use of tetracycline-class drugs, and the current recommended treatment reflects this, as doxycycline is most commonly prescribed.[55][46] This change in treatment recommendation coincided with a decrease in annual case-fatality rates (CFRs) from the 1980s on to the early 1990s.[56] Since then, the fatality rate has dropped to between 5 and 10%.[56] References
Further reading
External links
|
||||||||||||||||||||||||||||||