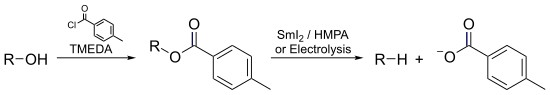
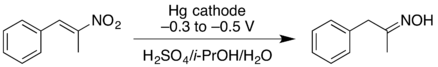
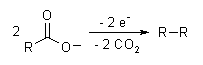Electrosynthesis
|
Read other articles:

ليوبولدو الثاني دوق توسكانا الأكبر (بالإيطالية: Leopoldo II d'Asburgo-Lorena) معلومات شخصية الميلاد 3 أكتوبر 1797(1797-10-03)فلورنسا الوفاة 29 يناير 1870 (72 سنة)روما مكان الدفن السرداب الإمبراطوري مواطنة دوقية توسكانا الكبرى اللقب دوق توسكانا الأكبر لون الشعر شعر أشقر الديانة الك�...

Launches made by the Geosynchronous Satellite Launch Vehicle Mk I and Mk II Liftoff of GSLV Mk. II F14 from Satish Dhawan Space Centre, carrying INSAT-3DS. This is a list of launches conducted by the Indian Space Research Organisation (ISRO) using Geosynchronous Satellite Launch Vehicle (GSLV) rockets. This list does not include LVM 3 (formerly known as GSLV Mk III) launches, which can be found here. Notable missions GSLV MK. I flight D1 Main article: GSLV This was the first developmental fli...

Historic church in New Hampshire, United States United States historic placeUnion ChurchU.S. National Register of Historic Places Union Church of South Wolfeboro, but now called the South Wolfeboro MeetinghouseShow map of New HampshireShow map of the United StatesLocationS. Main St., South Wolfeboro, New HampshireCoordinates43°33′47″N 71°10′38″W / 43.56306°N 71.17722°W / 43.56306; -71.17722Area1 acre (0.40 ha)Built1845 (1845)Architectural sty...
جزء من سلسلة مقالات حولعلم النفس تاريخ فروع خطوط عريضة المواضيع الرئيسية اللاقياسي السلوكي علم الوراثة السلوكي الحيوي المعرفي/معرفية المقارن عبر الثقافة الثقافي التنموي التمايزي التطوري التجريبي الحسابي العصبي الشخصية الإيجابي النفسي الديناميكي القياس النفسي الكمي ال...

Not to be confused with Beipu in Xincheng, Hualien. Place in Taiwan, Republic of ChinaBeipu Township北埔鄉 HoppoBeipu Township in Hsinchu CountyBeipu Township北埔鄉Coordinates: 24°39′50″N 121°4′5″E / 24.66389°N 121.06806°E / 24.66389; 121.06806CountryRepublic of ChinaProvinceTaiwanCountyHsinchuGovernment • TypeRural townshipArea • Total50.6676 km2 (19.5629 sq mi)Population (March 2023) • Total8,6...
Letak kota Larnaca Larnaca merupakan sebuah kota di Siprus. Kota ini letaknya di bagian selatan negara itu. Tepatnya di Distrik Larnaca. Pada tahun 2001, kota ini memiliki jumlah penduduk sebesar 72.000 jiwa. Wali kotanya ialah Andreas Moyseos. Bandar Udara Internasional Larnaca merupakan bandar udara terbesar di kota ini. Sejarah Pada zaman kuno, Larnaca dikenal sebagai tempat asal orang Kitim. Pranala luar Wikimedia Commons memiliki media mengenai Larnaca. Wikivoyage memiliki panduan wisata...

此條目可参照英語維基百科相應條目来扩充。 (2021年5月6日)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 约翰斯顿环礁Kalama Atoll 美國本土外小島嶼 Johnston Atoll 旗幟颂歌:《星條旗》The Star-Spangled Banner約翰斯頓環礁�...

The Exchequer Standards may refer to the set of official English standards for weights and measures created by Queen Elizabeth I (English units), and in effect from 1588 to 1825, when the Imperial units system took effect, or to the whole range of English unit standards maintained by the Court of the Exchequer from the 1200s, or to the physical reference standards physically kept at the Exchequer and used as the legal reference until the such responsibility was transferred in the 1860s, afte...

Ceramic tile cutters are used to cut ceramic tiles to a required size or shape. They come in a number of different forms, from basic manual devices to complex attachments for power tools.[1] Hand tools Beam score cutters, cutter boards First Tile Cutter Invented by Boada Brothers The ceramic tile cutter works by first scratching a straight line across the surface of the tile with a hardened metal wheel and then applying pressure directly below the line and on each side of the line on ...

American clergyman This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: John Michael McNamara – news · newspapers · books · scholar · JSTOR (August 2015) (Learn how and when to remove this message) John Michael McNamaraAuxiliary Bishop of WashingtonBishop McNamara High SchoolIn office1947-1960Other post(s)Titular...

Website editing and administration tool Microsoft Office FrontPageMicrosoft Office FrontPage 2003 running on Windows XPOriginal author(s)Vermeer TechnologiesDeveloper(s)MicrosoftInitial releaseNovember 1995; 28 years ago (1995-11) (as Vermeer FrontPage)Final release2003 (11.8164.8172) / September 17, 2007; 16 years ago (2007-09-17)[1] Operating systemMicrosoft WindowsTypeHTML editorLicenseProprietary Microsoft FrontPage (full name Microsoft Off...

Jaroslav Popovyč Jaroslav Popovyč allo Scheldeprijs 2015 Nazionalità Ucraina Altezza 175[1] cm Peso 71[1] kg Ciclismo Specialità Strada Termine carriera 2016 CarrieraSquadre di club 2000-2001Zoccorinese-Vellutex2002-2004 Landbouwkrediet2005-2007 Discovery Channel2008 Silence-Lotto2009 Astana2010-2011 RadioShack2012-2013 RadioShack2014-2016 TrekNazionale 2002-2013 UcrainaPalmarès Mondiali su strada Argento Plouay 2000 In line...

Aspect of the pandemic COVID-19 pandemic in the British Indian Ocean TerritoryDiseaseCOVID-19Virus strainSARS-CoV-2LocationBritish Indian Ocean TerritoryFirst outbreakWuhan, Hubei, ChinaArrival dateNovember 2020Confirmed cases5[1][2]Suspected cases‡0Recovered2Deaths0‡Suspected cases have not been confirmed by laboratory tests as being due to this strain, although some other strains may have been ruled out. Part of a series on theCOVID-19 pandemicin the United Kingdom, Brit...

American bookseller and retailer B&N redirects here. For the bank, see B&N Bank. Barnes & Noble BooksellersBarnes & Noble's current flagship store at Union Square, Manhattan, New York CityCompany typePrivateISINUS0677741094Industrybookselling PredecessorArthur Hinds & CompanyFounded1886; 138 years ago (1886) (as Arthur Hinds & Company) in New York City, U.S.FoundersCharles M. BarnesWilliam BarnesG. Clifford NobleLeonard Riggio[1][2 ...

Public radio station in Philadelphia WJAZ redirects here. For the former radio stations in Chicago, see WJAZ (Chicago). This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: WRTI – news · newspapers · books · scholar · JSTOR (March 2017) (Learn how and when to remove this message) WRTIPhiladelphia, PennsylvaniaBro...

1939 novel by John Steinbeck This article is about the novel. For other uses, see Grapes of Wrath (disambiguation). The Grapes of Wrath First edition coverAuthorJohn SteinbeckCover artistElmer HaderLanguageEnglishGenreNovelPublisherThe Viking Press-James LloydPublication dateApril 14, 1939[1]Publication placeUnited StatesPages464OCLC289946Dewey Decimal813.52 The Grapes of Wrath is an American realist novel written by John Steinbeck and published in 1939.[2] The book won t...

Pour les articles homonymes, voir Moreau. Christophe MoreauChristophe Moreau pendant le Tour de France 2009InformationsNaissance 12 avril 1971 (53 ans)VervinsNationalités suissefrançaiseSpécialité Courses par étapesÉquipes amateurs 1992Lions de Belfort1993CC Étupes1994VC Lyon-Vaulx-en-VelinÉquipes professionnelles 09.1994-12.1994Chazal-MBK-Köning (stagiaire)1995-2001Festina-Lotus2002-2005Crédit agricole2006-2007AG2R Prévoyance2008-2009Agritubel2010Caisse d'ÉpargneÉquipes di...

Fictional society in the Marvel Comics universe Hellfire ClubCover for X-Men: Legacy #210, art by David Finch.Publication informationPublisherMarvel ComicsFirst appearanceThe Uncanny X-Men #129 (January 1980)Created byChris Claremont John ByrneIn-story informationBase(s)VariousMember(s) Sebastian Shaw Emma Frost Harry Leland Jason Wyngarde Donald Pierce Tessa Selene Magneto Storm Shinobi Shaw Trevor Fitzroy Madelyne Pryor Sat-yr-9 Phoenix Sunspot Iron Monger Azazel The Hellfire Club is a fict...

رامون عزيز معلومات شخصية الميلاد 12 ديسمبر 1992 (العمر 31 سنة)أبوجا الطول 1.69 م (5 قدم 6 1⁄2 بوصة) مركز اللعب وسط الجنسية نيجيريا معلومات النادي النادي الحالي غرناطة الرقم 12 مسيرة الشباب سنوات فريق Future Pro Academy المسيرة الاحترافية1 سنوات فريق م. (هـ.) 2011–2016 ألميريا �...

British soldier and royal bastard (1799–1854) Lord Frederick FitzClarenceGCHLord Frederick FitzClarenceBorn9 December 1799Died30 October 1854(1854-10-30) (aged 54)Allegiance United KingdomService/branch British ArmyYears of service1814–1854RankLieutenant-GeneralCommandsBombay ArmyAwardsKnight Grand Cross of the Royal Guelphic OrderSpouse(s)Lady Augusta BoyleChildrenAugusta FitzClarenceWilliam FitzClarenceRelationsWilliam IV (father)Dorothea Jordan (mother) Lieutenant-General Lor...