Dimethylmagnesium
| |||||||||||||||||||||||||||||||||||||||||
Read other articles:

Ants that store food in living workers Myrmecocystus honeypot ants, showing the repletes or plerergates, their abdomens swollen to store honey, above ordinary workers Honeypot ants, also called honey ants, are ants which have specialized workers (repletes,[1] plerergates, or rotunds) that are gorged with food to the point that their abdomens swell enormously. Other ants then extract nourishment from them, through the process of trophallaxis. They function as living larders. Honeypot a...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Desember 2023. Tomanija ĐuričkoLahir(1914-02-16)16 Februari 1914Šabac, Serbia, YugoslaviaMeninggal31 Januari 1994(1994-01-31) (umur 79)Beograd, Serbia, YugoslaviaPekerjaanPemeranTahun aktif1955-1987 Tomanija Đuričko (16 Februari 1914 – ...

Château-du-Loir General informationLocationRue du Val de Loir 72500 Château-du-LoirSartheFranceElevation49 mOwned bySNCFOperated bySNCFPlatforms2Tracks2Other informationStation code87396606HistoryOpened19 July 1858Passengers2018159 432[1] Services Preceding station TER Pays de la Loire Following station Vaastowards Le Mans 25 Saint-Paterne-Racantowards Tours Gare de Château-du-Loir is a railway station serving the town Château-du-Loir, Sarthe department, western France. Services T...

Синелобый амазон Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:ЗавропсидыКласс:Пт�...

Frank AbagnaleFrank William Abagnale, Jr. pada tahun 2007LahirFrank William Abagnale Jr.27 April 1948 (umur 75)The Bronx, New York, U.S.Warga negaraUnited States, FrancePekerjaanSecure document consultantGugatan kejahatanAuto larceny, theft, forgery, fraudHukuman kriminal 4 months in a French prison 4 months in a Swedish prison 3 years, 3 months, and 7 days in a US federal prison 3 years in Great Meadow Correctional Facility, NY (age 17–20) Frank William Abagnale, Jr (lahir 27 April 1...

Voce principale: Unione Sportiva Dilettantistica Rivarolese 1919. Unione Sportiva RivaroleseStagione 1922-1923Sport calcio Squadra Rivarolese Prima Divisione10º posto nel girone B della Lega Nord. Retrocessa in Seconda Divisione. Si invita a seguire il modello di voce Questa pagina raccoglie i dati riguardanti l'Unione Sportiva Rivarolese nelle competizioni ufficiali della stagione 1922-1923. Indice 1 Stagione 2 Rosa 3 Risultati 3.1 Prima Divisione 3.1.1 Girone B 3.1.1.1 Girone di anda...

Historic federal government building in Washington DC, United States United States historic placeSupreme Court BuildingU.S. National Historic Landmark West Facade of the U.S. Supreme Court BuildingShow map of Central Washington, D.C.Show map of the United StatesLocation1 First Street, NortheastWashington, D.C., U.S.Coordinates38°53′26″N 77°0′16″W / 38.89056°N 77.00444°W / 38.89056; -77.00444Built1932–1935ArchitectCass Gilbert, Cass Gilbert Jr.Designated&#...

13th-century crusade against Catharism in southern France Albigensian CrusadePart of the CrusadesMassacre against the Albigensians by the CrusadersDateJuly 1209 – 12 April 1229 (19 Years)LocationLanguedoc, FranceResult Crusader victoryBelligerents Crusade: Crusader volunteers Episcopal Inquisition Dominican Order Kingdom of France Cathars County of Toulouse Viscounty of Béziers and Albi Crown of Aragon County of Foix Viscounty of Carcassonne Kingdom of EnglandComma...

1993 IAAF WorldIndoor ChampionshipsTrack events60 mmenwomen200 mmenwomen400 mmenwomen800 mmenwomen1500 mmenwomen3000 mmenwomen60 m hurdlesmenwomen4 × 400 m relaymenwomen3000 m walkwomen5000 m walkmenField eventsHigh jumpmenwomenPole vaultmenLong jumpmenwomenTriple jumpmenwomenShot putmenwomenCombined eventsPentathlonwomenHeptathlonmenvte The men's pole vault event at the 1993 IAAF World Indoor Championships was held on 12 and 13 March. Medalists Gold Silver Bronze Radion Gataullin ...

Period of Icelandic statehood from 1918 to 1944 Kingdom of IcelandKonungsríkið Ísland (Icelandic)Kongeriget Island (Danish)1918–1944 Flag Coat of arms Anthem: Ó Guð vors lands (O, God of Our Land)The Kingdom of Iceland in 1942StatusPersonal union with DenmarkCapitalReykjavíkCommon languagesIcelandic, DanishReligion Church of Iceland(state religion)GovernmentUnitary parliamentary constitutional monarchyKing • 1918–1944 Kristján X Regent • ...

この項目には、一部のコンピュータや閲覧ソフトで表示できない文字が含まれています(詳細)。 数字の大字(だいじ)は、漢数字の一種。通常用いる単純な字形の漢数字(小字)の代わりに同じ音の別の漢字を用いるものである。 概要 壱万円日本銀行券(「壱」が大字) 弐千円日本銀行券(「弐」が大字) 漢数字には「一」「二」「三」と続く小字と、「壱」「�...

British films released in 1933 Cinema of theUnited Kingdom List of British films British horror 1888–1919 1920s 1920 1921 1922 1923 19241925 1926 1927 1928 1929 1930s 1930 1931 1932 1933 19341935 1936 1937 1938 1939 1940s 1940 1941 1942 1943 19441945 1946 1947 1948 1949 1950s 1950 1951 1952 1953 19541955 1956 1957 1958 1959 1960s 1960 1961 1962 1963 19641965 1966 1967 1968 1969 1970s 1970 1971 1972 1973 19741975 1976 1977 1978 1979 1980s 1980 1981 1982 1983 19841985 1986 1987 1988 1989 1990...

Хип-хоп Направление популярная музыка Истоки фанкдискоэлектронная музыкадабритм-энд-блюзреггидэнсхоллджаз[1]чтение нараспев[англ.]исполнение поэзииустная поэзияозначиваниедюжины[англ.]гриотыскэтразговорный блюз Время и место возникновения Начало 1970-х, Бронкс, Н...

舞台となった若松港に立つ火野葦平(1953年) 主人公である火野の父、玉井金五郎(1936年頃) ポータル 文学 『花と竜』(はなとりゅう)は、1952年(昭和27年)4月から1953年(昭和28年)5月まで『読売新聞』に連載された火野葦平の長編小説である。 内容 明治中期から太平洋戦争後の北九州を舞台に、著者の父である玉井金五郎(若松の仲士・玉井組組長)と妻の�...
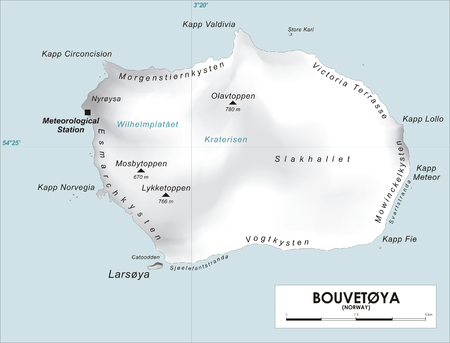
Promontory on Bouvet Island Map of Bouvetøya Cape Lollo (Norwegian: Kapp Lollo), located at 54°25′S 3°29′E / 54.417°S 3.483°E / -54.417; 3.483, is a cape which forms the northeastern extremity of Bouvetøya in Norway. It was first charted in 1898 by a German expedition under Carl Chun, and was recharted and named in December 1927 by a Norwegian expedition under Captain Harald Horntvedt.[1][2] See also Spiess Rocks References ^ Cape Lollo. Geogr...

17th c. Glasgow Town Clerk Mr. John SpreulBornSurname is sometimes spelled Spreull or Sprewll[1]1616GlasgowDied1690TitleMr. (being a graduate)SpouseCatherine Merchel (17 November 1640)[2]Children12+ John Spreul (born 1616[2]) was a town clerk in Glasgow who was educated at the University of Glasgow, where he completed his Master of Arts degree in 1635. His father was the Provost of Renfrew and an MP for Renfrew.[3] After university, he thought about becoming a ...

This article needs to be updated. Please help update this article to reflect recent events or newly available information. (May 2023) Political party in United States Congressional Constitution Caucus ChairmanNoneFoundersScott Garrett Virginia Foxx Rob BishopFoundedJanuary 3, 2005 (2005-01-03)[1]Political positionRight-wingSeats in the Senate0 / 100 Seats in the House26 / 435 WebsiteOfficial Caucus WebsitePolitics of United StatesPolitical partiesElections The...

Gran Premio del Giappone 1989 483º GP del Mondiale di Formula 1Gara 15 di 16 del Campionato 1989 Data 22 ottobre 1989 Luogo Circuito di Suzuka Percorso 5,859[1] km / 3,641 US mi Pista permanente Distanza 53[1] giri, 310,527[1] km/ 192,952 US mi Clima nuvoloso Risultati Pole position Giro più veloce Ayrton Senna Alain Prost McLaren - Honda in 1'38041[2] McLaren - Honda in 1'43506[1][3] (nel giro 43[1]) Podio 1. Alessandro Nannini...
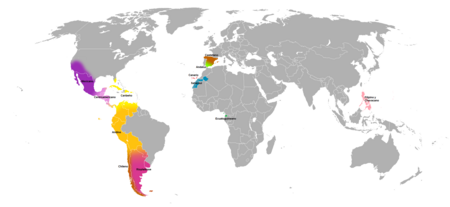
スペイン語の主な変種。 黄色がカリブ・スペイン語 カリブ・スペイン語(カリブ・スペインご、スペイン語: español caribeño)は、大アンティル諸島とカリブ海沿岸で有力なスペイン語の方言である。キューバ、ドミニカ共和国、プエルト・リコといった島の領域と、ベネズエラ、コロンビア、パナマといった大陸の領域で話される。 アンダルシア方言(港間での歴�...

Second-highest-ranking official of the US Senate For a list of presidents pro tempore of the Senate, see List of presidents pro tempore of the United States Senate. President pro tempore of the United States SenateSeal of the president pro temporeIncumbentPatty Murraysince January 3, 2023United States SenateStyle Madam President (when presiding) The Honorable (formal) SeatSenate chamber, United States Capitol, Washington, D.C.AppointerUnited States SenateTerm lengthAt the pleasure of the...
