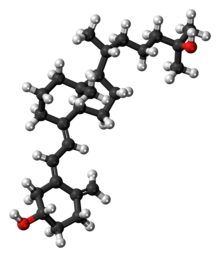
Calcifediol
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Michel OnfrayLahir1 Januari 1959EraFilsafat abad ke-20, abad ke-21KawasanFilsafat barat, filsafat kontinentalAliranAteisme, Hedonisme, PostanarkismeMinat utamaAteisme, agama, etika, mazhab Kirene, Epikureanisme, kesenangan, sejarah filsafat, materialisme, estetika, bioetika Dipengaruhi Demokritus, Aristippus, Epikouros, Friedrich Nietzsche, anarkisme individualis, Georges Bataille, Michel de Montaigne, Han Ryner, Gilles Deleuze, Pierre Hadot, Julien Offray de La Mettrie, Michel Foucault...

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Oktober 2022. Izmir SmajlajInformasi pribadiLahir29 Maret 1993 (umur 30)Shkodër, AlbaniaTinggi190 m (623 ft 4 in)Berat80 kg (176 pon) OlahragaOlahragaAtletikLombaLompat jauhKlubTironaDilatih olehGjergj Ruli Rekam medali Atletik putra M...

Globalisasi Budaya Demokratis Ekonomi Historis Garis besar Istilah Portal Studi Commons Kategorilbs Fisikawan teori Albert Einstein adalah contoh pelarian modal manusia akibat perubahan politik. Ia pindah ke Amerika Serikat untuk menghindari penindasan Nazi. Pelarian modal manusia, kadang disebut pengurasan keterampilan (Inggris: brain draincode: en is deprecated ), mengacu pada perpindahan orang-orang pintar dan terdidik demi mencari upah atau kondisi kerja yang lebih baik sehingga negara as...

Sleeping disorder For the normal sleep behavior of body paralysis during dreams, see Rapid eye movement sleep. Night demon redirects here. For the American heavy metal band, see Night Demon (band). Medical conditionSleep paralysisThe Nightmare by Swiss artist Henry Fuseli (1781) is thought to be a depiction of sleep paralysis perceived as a demonic visitation.Specialty Psychiatry sleep medicine Symptoms Awareness but an inability to move during waking or falling asleep hallucinations[1 ...

Pour les articles homonymes, voir Dioné. Dioné Déesse de la mythologie grecque Dioné sur son trône. Caractéristiques Nom Grec ancien Διώνη (Diốnê) Résidence Olympe Lieu d'origine Grèce antique Période d'origine Grèce archaïque Groupe divin Les Océanides et les divinités marines Parèdre Zeus Culte Temple(s) Sanctuaire oraculaire de Dodone Mentionné dans Iliade d'Homère ; Théogonie d'Hésiode ; Bibliothèque d'Apollodore ; Fables d'Hygin Famille Père Oc...

Subspecies of honey bee Apis mellifera mellifera Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Arthropoda Class: Insecta Order: Hymenoptera Family: Apidae Genus: Apis Species: A. mellifera Subspecies: A. m. mellifera Trinomial name Apis mellifera melliferaLinnaeus, 1758[1] Synonyms[2] Apis mellifica germanica (Pollmann 1879) Apis mellifica nigrita (Lucas 1882) Apis mellifica mellifica variety lehzeni (Buttel-Reepen 1906) Apis mellifica mellifi...
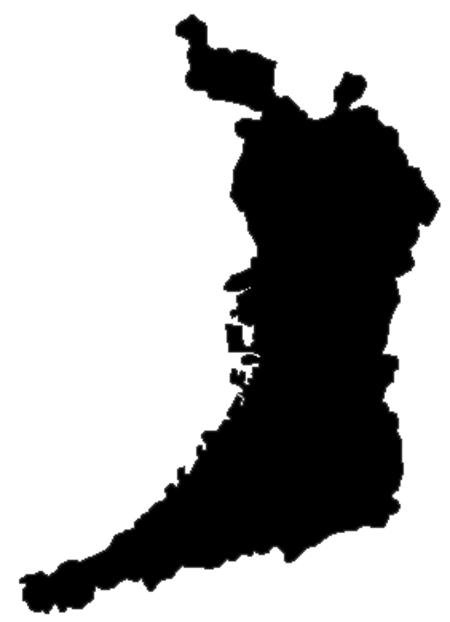
This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Senboku District, Osaka – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this template message) Location of Senboku District in Osaka Senboku (泉北郡, Senboku-gun) is a district located in Osaka Prefecture, Japan. As of 2009, the dis...

SV Darmstadt 1898Calcio Die Lilien (i gigli) Segni distintivi Uniformi di gara Casa Trasferta Terza divisa Colori sociali Blu, bianco Simboli Giglio Dati societari Città Darmstadt Nazione Germania Confederazione UEFA Federazione DFB Campionato Bundesliga Fondazione 1898 Presidente Klaus Rüdiger Fritsch Allenatore Torsten Lieberknecht Stadio Stadion am Böllenfalltor(17.000 posti) Sito web www.sv98.de Palmarès Titoli nazionali 2 Bundesliga 2 Si invita a seguire il modello di voce Lo ...

† Человек прямоходящий Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:Синапсиды�...

List of ways of hiding objects or animals in plain sight Camouflage is the concealment of animals or objects of military interest by any combination of methods that helps them to remain unnoticed. This includes the use of high-contrast disruptive patterns as used on military uniforms, but anything that delays recognition can be used as camouflage. Camouflage involves deception, whether by looking like the background or by resembling something else, which may be plainly visible to observers.&#...

OpenStackTipesoftware system dan Komputasi awan Versi pertama21 Oktober 2010; 13 tahun lalu (2010-10-21)Versi stabil 2023.2 Bobcat (4 Oktober 2023) GenreKomputasi awanLisensiApache License 2.0Karakteristik teknisSistem operasiLintas platformBahasa pemrogramanPython Sumber kode Debianopenstack-cloud-services Informasi tambahanSitus webwww.openstack.orgBlogBlog oficial Pelacakan kesalahanLaman pelacakan Sunting di Wikidata • Sunting kotak info • L • BBantuan pengguna...

Not to be confused with the Dexter Grist Mill in Sandwich, Massachusetts. United States historic placeDexter Grist MillU.S. National Register of Historic Places Show map of MaineShow map of the United StatesLocationDexter, MaineCoordinates45°1′23″N 69°17′29″W / 45.02306°N 69.29139°W / 45.02306; -69.29139Area1 acre (0.40 ha)Built1854 (1854)Built byCaleb B. CurtisArchitectural styleVernacular IndustrialNRHP reference No.75000104[1...

هذه المقالة عن دراجة هوائية. لاستخدامات ومصطلحات أخرى، طالع دراجة (توضيح). دراجة هوائيةمعلومات عامةصنف فرعي من velocipede (en) مركبة ثنائية العجلةمعدات رياضية الاستعمال القائمة ... نقل[1] سباق الدراجات الهوائية ركوب الدراجات التجول على الدراجة organized bicycle touring (en) individual...

Kim Hyun-joong awards and nominationsKim at SS501's concert in Hong Kong, 2009Awards and nominationsAward Wins NominationsTotals[a]Wins34Nominations40Note ^ Certain award groups do not simply award one winner. They recognize several different recipients, have runners-up, and have third place. Since this is a specific recognition and is different from losing an award, runner-up mentions are considered wins in this award tally. For simplification and to avoid errors, each award in this...

U.S. federal surveying and mapping agency Not to be confused with Office of Coast Survey, National Ocean Service, United States Coast and Geodetic Survey, or United States Geological Survey. The National Geodetic Survey (NGS) is a United States federal agency based in Washington, D.C. that defines and manages a national coordinate system, providing the foundation for transportation and communication, mapping and charting, and a large number of science and engineering applications. Since its f...

German WWII underground rocket factory MittelwerkNordhausen, Thuringia Interior of the tunnelsMittelwerkCoordinates51°32′05″N 10°45′04″E / 51.5348°N 10.751°E / 51.5348; 10.751TypeSubterranean bunkerSite informationOpen tothe publicMuseum in the southern part of Tunnel A[1]Site historyBuiltCompleted 1943Built byMittelwerk GmbHIn use1943–1945 Mittelwerk ([ˈmɪtl̩.vɛʁk]; German for Central Works) was a German World War...

بون نوفال Bonne-NouvelleBonne Nouvelle (بالفرنسية) معلومات عامةالمدينة باريسالتقسيم الإداري الدائرة الثانية في باريس — الدائرة التاسعة في باريس — الدائرة العاشرة في باريس البلد فرنسا شبكة المواصلات مترو باريس المالك RATPالإدارة الهيئة المستقلة للنقل في باريس الخطوط الخط 8 لمترو با�...

This page is an archive of past discussions. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. Archives 2023;Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2022;Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2021;Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2020;Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2019;Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2018;Jan Feb Mar Apr May Jun Jul Aug Sep...

Štúrovo Párkány Kota Pemandangan Jembatan Mária Valéria yang menghubungkan kota Štúrovo dengan Esztergom Negara Slovakia Region Nitra Kabupaten Nové Zámky Sungai Donau, Hron Elevasi 111 m (364 ft) Koordinat Area 13,13 km2 (5 sq mi) Population 10.465 (2016-12-31[1]) Density 797 / km² (2.064 / sq mi) First mentioned 1075 Mayor Eugen Szabó Zona waktu CET (UTC+1) - summer (DST) CEST (UTC+2) Kode pos 943 01 Prefiks telepon 421-3...

La cible humaine Données clés Titre original The Gunfighter Réalisation Henry King Scénario William Bowers et André de Toth Acteurs principaux Gregory PeckHelen WestcottKarl MaldenJean Parker Sociétés de production Twentieth Century-Fox Corporation Pays de production États-Unis Genre Western Durée 85 minutes Sortie 1950 Pour plus de détails, voir Fiche technique et Distribution. modifier La Cible humaine (titre original : The Gunfighter) est un film américain réalisé par He...