|
Protactinyo
| Protactinium
|
91Pa
|
|
|
| Panagway
|
bright, silvery metallic luster
Payl:Protactinium.jpg
|
| Kinatibuk-ang mga kinaiya
|
| Ngalan, simbolo, kaiphan
|
protactinium, Pa, 91
|
| Paglitok
|
[[Help:Pronunciation respelling key|Plantilya:Smallcaps all-tak-Plantilya:Smallcaps all-ee-əm]]
|
| Kategoriyang elemento
|
aktinido
|
| Group, period, block
|
n/a, 7, f
|
| Gibug-aton sa atomo
|
231.03588
|
| Kontorno sa elektron
|
[Rn] 5f2 6d1 7s2
2, 8, 18, 32, 20, 9, 2
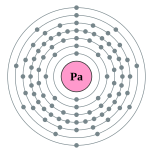 Electron shells of protactinium (2, 8, 18, 32, 20, 9, 2) Electron shells of protactinium (2, 8, 18, 32, 20, 9, 2) |
| History
|
| Prediction
|
Dmitri Mendeleev (1869)
|
| Pagkadiskobre
|
William Crookes (1900)
|
| First isolation
|
William Crookes (1900)
|
| Named by
|
Otto Hahn and Lise Meitner (1917–8)
|
| Physical properties
|
| Phase
|
magahi
|
| Density (near r.t.)
|
15.37 g·cm−3
|
| Melting point
|
1841 K, 1568 °C, 2854 °F
|
| Boiling point
|
? 4300 K, ? 4027 °C, ? 7280 °F
|
| Heat of fusion
|
12.34 kJ·mol−1
|
| Heat of vaporization
|
481 kJ·mol−1
|
| Atomic properties
|
| Oxidation states
|
2, 3, 4, 5
(weakly basic oxide)
|
| Electronegativity
|
1.5 (Pauling scale)
|
| Ionization energies
|
1st: 568 kJ·mol−1
|
| Atomic radius
|
163 pm
|
| Covalent radius
|
200 pm
|
| Miscellanea
|
| Crystal structure
|
tetragonal[1]
|
| Magnetic ordering
|
paramagnetic[2]
|
| Electrical resistivity
|
(0 °C) 177 nΩ·m
|
| Thermal conductivity
|
47 W·m−1·K−1
|
| CAS registry number
|
7440-13-3
|
| Most stable isotopes
|
| Main article: Isotopes of protactinium
|
|
|
| · r
|
Ang protactinyo (Iningles protactinium) mao ang elementong kimiko sa talaang peryodiko nga may simbolo nga Pa ug kaiphan nga atomik 91. Ang protactinyo mao ang aktinido.
Ang mga gi basihan niini
- ↑ Donohue, J. (1959). "On the crystal structure of protactinium metal". Acta Crystallographica. 12 (9): 697. doi:10.1107/S0365110X59002031.
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
Galeriya sa hulagway
|
|