Sextuple bond
|
Read other articles:

Questa voce o sezione sull'argomento militari britannici non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Segui i suggerimenti del progetto di riferimento. George RookeL'Ammiraglio ritratto da Michael DahlNascitaCanterbury, 1650 MorteCanterbury, 24 gennaio 1709 Dati militariPaese servitoRegno d'Inghilterra Forza armata Royal Navy Anni di ...

سترومبولي الإحداثيات 38°48′14″N 15°13′24″E / 38.804°N 15.2233°E / 38.804; 15.2233 تقسيم إداري البلد إيطاليا[1] التقسيم الأعلى ليبر خصائص جغرافية المساحة 12.6 كيلومتر مربع عدد السكان عدد السكان 450 (1 يناير 2001) الكثافة السكانية 35.71 نسمة/كم2 معلومات أخر...

EumenesNama dalam bahasa asli(grc) Εὐμένης BiografiKelahiran362 SM Cardia (en) Kematian316 SM (45/46 tahun)Q3756426 Strategos Satrap KegiatanPekerjaanPerwira militer, personel militer dan satrap KesetiaanKekaisaran Makedonia Pangkat militerlaksamana KeluargaPasangan nikahArtonis (en) Barsine Eumenes dari Cardia (/juːˈmɛniːz/; Yunani: Εὐμένης; skt. 362 – 316 SM) merupakan seorang jenderal dan sarjana Yunani. Ia berpartisipasi di dalam P...

Strada statale 502di CingoliLocalizzazioneStato Italia Regioni Marche Province Ancona Macerata DatiClassificazioneStrada statale InizioJesi Fineex SS 78 presso Pian di Pieca Lunghezza73,100[1] km Provvedimento di istituzioneD.M. 18/02/1966 - G.U. 92 del 15/04/1966[2] GestoreTratte ANAS: nessuna (dal 2001 la gestione è passata alla Provincia di Ancona e alla Provincia di Macerata)Dal 2017 è di nuovo competenza ANAS Manuale La ex strada statale 502 di Cingo...

San Maurizio d'Opaglio commune di Italia Tempat Negara berdaulatItaliaRegion di ItaliaPiedmontProvinsi di ItaliaProvinsi Novara NegaraItalia PendudukTotal2.955 (2023 )GeografiLuas wilayah8,51 km² [convert: unit tak dikenal]Ketinggian370 m Berbatasan denganMadonna del Sasso (en) Orta San Giulio Pella Pogno Gozzano SejarahSanto pelindungSanto Mauritius Informasi tambahanKode pos28017 Zona waktuUTC+1 UTC+2 Kode telepon0322 ID ISTAT003133 Kode kadaster ItaliaI025 Lain-lainSitus we...

Main article: Lists of figures in Germanic heroic legend Starkad as illustrated on Carta Marina (1539) by Olaus Magnus. P Figure Names in medieval languages Historical origin Name meaning Relationships Early and English Attestations Norse Attestations German Attestations Patavrid Latin: Patavrid The first element pata from PGmc *badu (battle),[1] the second element PGmc *friþu (peace).[2] Hagen/Högni1's nephew, Hagen unsuccessfully tries to prevent him from fighting against...

هذه المقالة تحتاج للمزيد من الوصلات للمقالات الأخرى للمساعدة في ترابط مقالات الموسوعة. فضلًا ساعد في تحسين هذه المقالة بإضافة وصلات إلى المقالات المتعلقة بها الموجودة في النص الحالي. (مارس 2018) مقاطعة بلايني الإحداثيات 43°23′N 113°59′W / 43.39°N 113.98°W / 43.39; -113.98&...

National Historic Landmark University of Wisconsin–Madison ArboretumUniversity of Wisconsin–Madison ArboretumTypeArboretumLocation1207 Seminole Hwy., Madison, WisconsinCoordinates43°02′29″N 89°25′50″W / 43.041425°N 89.430652°W / 43.041425; -89.430652Area1,260 acres (510 ha)CreatedApril 26, 1932 (1932-04-26)Operated byUniversity of Wisconsin–MadisonUniversity of Wisconsin–Madison ArboretumU.S. National Register of Historic Pl...

Medieval kingdom in Scotland Political centres in Scotland in the early Middle Ages The Kingdom of Alba (Latin: Scotia; Scottish Gaelic: Alba) was the Kingdom of Scotland between the deaths of Donald II in 900 and of Alexander III in 1286. The latter's death led indirectly to an invasion of Scotland by Edward I of England in 1296 and the First War of Scottish Independence. Alba included Dalriada, but initially excluded large parts of the present-day Scottish Lowlands, which were then divided ...

University in Montreal, Quebec, Canada UdeM redirects here. For other uses, see UdeM (disambiguation). Not to be confused with Université du Québec à Montréal. University of MontrealUniversité de Montréal (French)Latin: Universitas Montis RegiiFormer nameUniversité Laval à MontréalMottoFide splendet et scientia (Latin)Motto in EnglishIt shines by faith and knowledgeTypePublicEstablished1878; 146 years ago (1878)Academic affiliationAUF, IFPU, Universities Canada...

مقلة العين الاسم العلميBulbus oculi رسم تخطيطي للعين البشرية. تفاصيل الشريان المغذي شرايين هدبية أمامية، شرايين هدبية خلفية طويلة، الشرايين الهدبية الخلفية القصيرة جزء من عين معرفات غرايز ص.1006 [عدل في ويكي بيانات ] تعديل مصدري - تعديل مقلة العين[1] (بالإنجليزية: Bu...

Type of road intersection Right-in/right-out (RIRO) and left-in/left-out (LILO) refer to a type of three-way road intersection where turning movements of vehicles are restricted. A RIRO permits only right turns and a LILO permits only left turns. Right-in and left-in refer to turns from a main road into an intersection (or a driveway or parcel); right-out and left-out refer to turns from an intersection (or a driveway or parcel) to a main road.[1][2][3] RIRO is typical...

Firearm part surrounding the trigger to hinder accidental discharge Trigger guard of a SG 550 rifle A trigger guard is a protective loop surrounding the trigger of a firearm designed to prevent unwanted contact with the trigger, which may cause an accidental discharge.[1] Other devices that use a trigger-like actuator mechanism, such as inhalers, crossbows and power tools, may also have trigger guards. On rifles with a bottom metal, the trigger guard is often incorporated as part of t...

This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Air Naval Gunfire Liaison Company – news · newspapers · books · scholar · JSTOR (September 2019) (Learn how and when to remove this message) Air Naval Gunfire Liaison Companies(ANGLICO)An ANGLICO team operates from a rooftop during the Iraq War.ActiveDecember, ...

Bambini che lavorano su un fonogramma alfabetico mobile. Il metodo Montessori è un sistema educativo sviluppato dalla pedagogista Maria Montessori, praticato in circa 60 000 scuole in tutto il mondo (con maggiore concentrazione negli Stati Uniti, in Germania, nei Paesi Bassi e nel Regno Unito), al servizio dei bambini e ragazzi compresi nella fascia di età dalla nascita fino a diciotto anni.[1] La pedagogia montessoriana si basa sull'indipendenza, sulla libertà di scelta del p...

不可逆性电穿孔(英語:irreversible electroporation,缩写:IRE)是透过极其短但强力的电场使得细胞膜上产生永久奈米孔的一种组织消融技术,透过扰动细胞稳态以让细胞死亡。这种手段导致细胞凋亡而不是其他基于热融、辐射的消融技术造成的细胞坏死。不可逆性电穿孔技术的主要用途是在需要维护重要细胞外基质、血流、神经的部位进行肿瘤消融。这个技术目前正在临床试�...

Kronik Burung Pegas Sampul edisi pertama di IndonesiaPengarangHaruki MurakamiJudul asliねじまき鳥クロニクルNejimakitori KuronikuruPenerjemahJay RubinNegaraJepangBahasaJepangDiterbitkan Shinchosha (JP) 1997 (Vintage) (US) Mei 2019 KPG (ID) Jenis mediaPrint (Hardcover)Halaman 925 (ID) 607 (JP) ISBNISBN 0-679-77543-9OCLC39915729 Kronik Burung Pegas (ねじまき鳥クロニクルcode: ja is deprecated , Nejimakitori Kuronikuru) adalah novel karangan Haruki Murakami yang terbi...
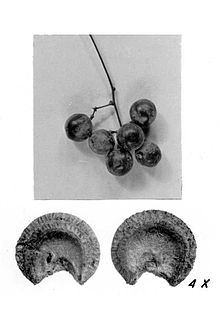
Genus of flowering plants Menispermum Fruit and seed of Menispermum canadense Scientific classification Kingdom: Plantae Clade: Tracheophytes Clade: Angiosperms Clade: Eudicots Order: Ranunculales Family: Menispermaceae Subfamily: Menispermoideae Genus: MenispermumL. Species See text Menispermum (moonseed) is a small genus of deciduous climbing woody vines in the moonseed family (Menispermaceae). Plants in this genus have small dioecious flowers, and clusters of small grape-like drupes.[1...

Villon's WifeOriginal Japanese Poster.SutradaraKichitaro NegishiDitulis olehYōzō TanakaPenata musikTakashi YoshimatsuSinematograferTakahide ShibanushiPenyuntingAkimasa KawashimaDistributorTohoTanggal rilis 10 Oktober 2009 (2009-10-10) Durasi114 min.NegaraJapanBahasaJapanese Villon's Wife (ヴィヨンの妻 〜桜桃とタンポポ〜code: ja is deprecated , Viyon No Tsuma - ōtō to tanpopo) adalah film Jepang produksi tahun 2009 yang disutradarai oleh Kichitaro Negishi, berdasa...

Death Note (film 2016) beralih ke halaman ini. Untuk film Tiongkok 2016, lihat The Death Note. Artikel ini bukan mengenai New World (Death Note). Death Note: Light Up the New WorldPosterSutradaraShinsuke SatoBerdasarkanDeath Noteoleh Tsugumi Ohba (cerita) dan Takeshi Obata (gambar)PemeranMasahiro Higashide Sosuke Ikematsu Masaki Suda Erika Toda Rina Kawaei Mina Fujii Nakamura Shidō II Sota AoyamaPerusahaanproduksiWarner Bros., NTVDistributorWarner Bros.Tanggal rilis 29 Oktober 2016 (201...


