Serotonin–dopamine reuptake inhibitor
|
Read other articles:

BremenflyBerkas:Bremenfly logo.jpg IATA ICAO Kode panggil 8B tumpang tindih dengan Business air BFY BORGWARDT Didirikan2008Mulai beroperasi2009Berhenti beroperasi2010Pusat operasiBandar Udara Berlin Schönefeld[1]Armada2 (saat penutupan)Kantor pusatSchönefeld, JermanSitus webwww.bremenfly.com Bremenfly Boeing 737-400 di Bandar Udara Berlin Schönefeld (2010). Bremenfly GmbH adalah maskapai penerbangan sewaan Jerman yang berbasis di Schönefeld, Jerman.[2] Tujuan Jerman ...

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (نوفمبر 2019) دوري ماكاو لكرة القدم 2014 تفاصيل الموسم دوري ماكاو لكرة القدم البلد الصين البطل نادي بنفيكا دي ماكا...

The signed copy of the Declaration, engrossed by Timothy Matlack, is now badly faded. It is on display in the Charters of Freedom rotunda at the National Archives in Washington, DC. The physical history of the United States Declaration of Independence spans from its original drafting in 1776 into the discovery of historical documents in modern time. This includes a number of drafts, handwritten copies, and published broadsides. The Declaration of Independence states that the Thirteen Colonie...

Gereja Kristus di Indonesia Gereja Kristus Di Indonesia (GKDI) Negara Indonesia Aliran non-denominasi Website www.gkdi.org Diarsipkan 2023-07-22 di Wayback Machine. Sejarah Didirikan 1990 (sebagai Jemaat Kristus Penginjilan Nusantara), 2001 (sebagai Gereja Kristus Di Indonesia) Afiliasi International Churches of Christ www.disciplestoday.org Diarsipkan 2020-11-01 di Wayback Machine. SEA Church www.seachurchesmedia.org Diarsipkan 2023-05-28 di Wayback Machine. HOPE Worldwide www.hopeww....

Place in Northern, IsraelBeit Yosef בֵּית יוֹסֵףBeit YosefShow map of Jezreel Valley region of IsraelBeit YosefShow map of IsraelCoordinates: 32°33′31″N 35°33′6″E / 32.55861°N 35.55167°E / 32.55861; 35.55167Country IsraelDistrictNorthernCouncilValley of SpringsAffiliationMoshavim MovementFounded1937Population (2022)[1]493 Beit Yosef (Hebrew: בֵּית יוֹסֵף) is a moshav in the northern Israel's Beit She'an Valley....

Szentendre IslandNative name: Szentendrei-szigetThe north end of Szentendre Island is visible on the left of this aerial view of the Danube BendSzentendre IslandGeographyLocationDanube RiverCoordinates47°44′50″N 19°06′32″E / 47.74722°N 19.10889°E / 47.74722; 19.10889AdministrationHungary Szentendre Island (Hungarian: Szentendrei-sziget) is an island in the Danube River between the Danube Bend and Budapest in Hungary. The island is flanked by the Szentendre ...

Mukjizat AllahGenre Drama Religi PembuatMultivision PlusDitulis olehAgam SuhartoSkenarioAdi Eka PrasetyaCeritaRagil SundaryantoSutradara Ahmad Yusuf Eddi Prasetya Irwin Mula Limbong Pengarah kreatifRaakhee PunjabiPemeran Anjasmara Krisdayanti Primus Yustisio Jihan Fahira Ramzi Dimas Seto Bunga Citra Lestari Ibnu Jamil Ayu Diah Pasha Billy Boedjanger Hj. Mursidah Misye Arsita Yana Zein Dicky Suprapto Arhinza Lagu pembukaAssalamu'alaikum oleh RaihanLagu penutupAssalamu'alaikum oleh RaihanPenat...

Disambiguazione – Se stai cercando altri significati, vedi The Amazing Spider-Man (disambigua). The Amazing Spider-Manserie regolare a fumetti The Amazing Spider-Man n. 1 nella versione italiana Lingua orig.inglese PaeseStati Uniti TestiStan Lee, Gerry Conway, Archie Goodwin, Len Wein, Bill Mantlo, David Michelinie, J.M. DeMatteis, Tom DeFalco, Dan Slott DisegniSteve Ditko EditoreMarvel Comics 1ª edizione(vol. 1) Marzo 1963 – Novembre 19...

House elections for the 77th U.S. Congress 1940 United States House of Representatives elections ← 1938 November 5, 1940[a] 1942 → All 435 seats in the United States House of Representatives218 seats needed for a majority Majority party Minority party Leader Sam Rayburn Joseph Martin Party Democratic Republican Leader since September 16, 1940 January 3, 1939 Leader's seat Texas 4th Massachusetts 14th Last election 262 seats ...
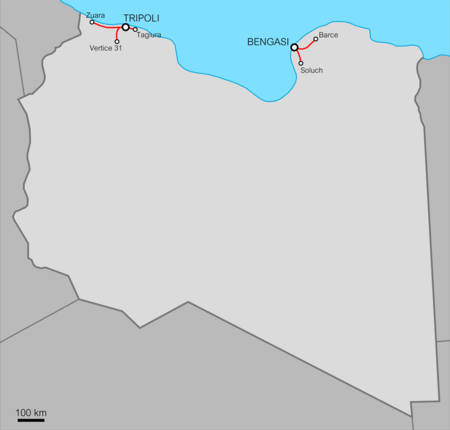
Questa voce sugli argomenti linee ferroviarie e trasporti in Libia è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Bengasi-BarceStati attraversati Libia InizioBengasi FineBarce Attivazione1914 (Bengasi–Benina)1916 (Benina–Regima)1926 (Regima–el-Abiar)1927 (el-Abiar–Barce) Soppressione1965 Precedenti gestoriFS? Lunghezza108 km Scartamento950 mm Elettrificazioneno Ferrovie Modifica dati su Wikidata · Manuale La ferrovia Beng...

Snowy RoadPoster teatrikalNama lainHangul눈길 Alih Aksara yang DisempurnakanNungil SutradaraLee Na-jeongProduserHam Yeong-hunDitulis olehYoo Bo-raPemeranKim Sae-ron Kim Hyang-giPenata musikNam Hye-seungSinematograferPark SungPenyuntingKim Young-juPerusahaanproduksiKBSat9 FilmsDistributorCGV ArthouseTanggal rilis Mei 2015 (2015-05) (JIFF) 01 Maret 2017 (2017-03-01) Durasi122 menitNegaraKorea SelatanBahasaKoreanJepangPendapatankotorUS$866,692[1] Snowy Road (H...

Species of bird Eurasian wigeon Male (rear) and female (front) Calls recorded in Dorset Conservation status Least Concern (IUCN 3.1)[1] Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Class: Aves Order: Anseriformes Family: Anatidae Genus: Mareca Species: M. penelope Binomial name Mareca penelope(Linnaeus, 1758) Synonyms Anas penelope Linnaeus, 1758 The Eurasian wigeon or European wigeon (Mareca penelope), also known as the widgeon or the wige...

American lawyer and politician Sean Reyes21st Attorney General of UtahIncumbentAssumed office December 30, 2013GovernorGary HerbertSpencer CoxPreceded byJohn Swallow Personal detailsBorn (1971-02-16) February 16, 1971 (age 53)[1]Political partyRepublicanSpouseSaysha FawsonChildren6EducationBrigham Young University (BA)University of California, Berkeley (JD)SignatureWebsiteOfficial website Sean David Reyes (born February 16, 1971) is an American lawyer and politician who has b...

1805 battle during the War of the Third Coalition Battle of DonauwörthPart of the War of the Third CoalitionCrossing of the Danube near Donauwörthby Wilhelm von Kobell, 1807Date7 October 1805LocationDonauwörth, Electorate of Bavaria, Holy Roman Empire48°41′47″N 10°48′00″E / 48.6964°N 10.8001°E / 48.6964; 10.8001Result French victoryBelligerents First French Empire Austrian EmpireCommanders and leaders Joachim Murat Jean-de-Dieu Soult Michael ...

Сельское поселение России (МО 2-го уровня)Новотитаровское сельское поселение Флаг[d] Герб 45°14′09″ с. ш. 38°58′16″ в. д.HGЯO Страна Россия Субъект РФ Краснодарский край Район Динской Включает 4 населённых пункта Адм. центр Новотитаровская Глава сельского пос�...

Servicio Forestal de los Estados Unidos Logo del U.S. Forest Service. LocalizaciónPaís Estados UnidosInformación generalJurisdicción Gobierno Federal de Estados UnidosTipo fuerza del orden del Gobierno de Estados Unidos, agencia federal de Estados Unidos, forestry agency y organizaciónSede Sidney R. Yates Federal BuildingOrganizaciónDirección Abigail R. Kimbell (Chief of the US Forest Service)Composición Pacific Northwest Research StationDepende de Departamento de AgriculturaDepe...

Le Nebraska est un État des États-Unis depuis le 1er mars 1867. Cette page dresse la liste des deux sénateurs qui représentent l'État au Congrès des États-Unis depuis de cette date. Deb Fischer (R), sénatrice depuis 2013. Pete Ricketts (R), sénateur depuis 2023. Sénateurs de classe I no Sénateur Parti Mandat Notes 1er Thomas Tipton Parti républicain 1er mars 1867 – 4 mars 1875(8 ans et 3 jours) Élu en 1867.Réélu en 1869. 2e Algernon Paddock Parti républicain ...

This is the talk page for discussing WikiProject Puerto Rico and anything related to its purposes and tasks. Put new text under old text. Click here to start a new topic. New to Wikipedia? Welcome! Learn to edit; get help. Assume good faith Be polite and avoid personal attacks Be welcoming to newcomers Seek dispute resolution if needed ShortcutsWT:PURWT:WPPR This project page does not require a rating on Wikipedia's content assessment scale.It is of interest to the following WikiProjects:Cou...

جيرارد ديلبيكي معلومات شخصية الميلاد 1 سبتمبر 1898 الوفاة سنة 1983 بلجيكا مركز اللعب لاعب وسط الجنسية بلجيكا المسيرة الاحترافية1 سنوات فريق م. (هـ.) 1923–1933 كلوب بروج المنتخب الوطني 1930 بلجيكا 1 (0) 1933–1934 كلوب بروج 1939–1945 كلوب بروج المواقع مُعرِّف الاتحاد الدولي لك...

2009 UK local government election 2009 Bristol City Council election ← 2007 4 May 2009 2010 → 23 of 70 seats (One Third) to Bristol City Council36 seats needed for a majority First party Second party Third party Party Liberal Democrats Conservative Labour Seats won 36 17 16 Seat change 4 4 8 Fourth party Party Green Seats won 1 Seat change 2009 local election results in Bristol Council control before election No Overall Con...


