Relugolix
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

M4 Kadıköy–KartalStasiun YenisahraIkhtisarJenisAngkutan CepatSistemMetro İstanbulStatusDibuka (3 stasiun masih dibangun)Lokasiİstanbul, TurkiTerminusKadıköyKartalStasiun16 (3 lebih masih dibangun)Layanan1Penumpang harian134,524 (2013)OperasiDibuka17 Agustus 2012; 11 tahun lalu (2012-08-17)PemilikMunisipalitas Metropolitan İstanbulOperatorİstanbul Ulaşım A.Ş.DepoMaltepeRangkaian144 CAF[1]Data teknisPanjang lintas217 km (135 mi)Jenis rel2Lebar sepur1.435 ...

RFK RacingPemilikJack RoushJohn W. Henry (Fenway Sports Group) Brad KeselowskiKantor pusatConcord, North CarolinaSeriNASCAR Cup SeriesPembalap6. Brad Keselowski 17. Chris BuescherSponsor6. Kohler Generators, Violet Defense, Fastenal, Castrol, Wyndham Rewards17. Fastenal, Wyndham Rewards, ITsavvy, Fifth Third Bank, Castrol, Violet DefensePabrikanFord,Berdiri1988Sejarah dalam ajang NASCARLomba pertamaCup Series:1988 Daytona 500 (Daytona)Xfinity Series:1992 Goody's 300 (Daytona)Camping World Tru...
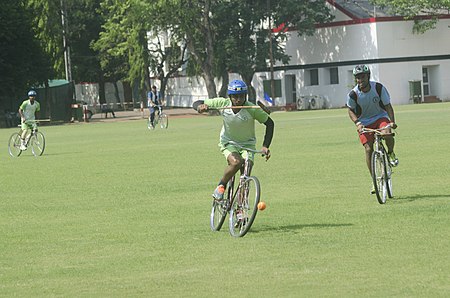
Team sport originating in Ireland; related to polo but played on bicycles Not to be confused with Cycle ball. Cycle poloBike polo match in BudapestHighest governing bodyInternational Bicycle Polo Federation, North American Bike Polo Association, European Hardcourt Bike Polo AssociationFirst playedOctober 1891 - County Wicklow, Ireland. (Rathclaren Rovers V Ohne Hast Cycling Club)CharacteristicsTeam membersFive or ThreeTypeTeam sportEquipmentBicycle, Mallet, BallPresenceOlympicLondon, 190...

British record label This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Fire Records UK – news · newspapers · books · scholar · JSTOR (September 2020) (Learn how and when to remove this message) Fire RecordsFounded1986FounderJohnny Waller, Clive SolomonGenreAlternative rock, indie rockCountry of originUnit...

Questa voce sull'argomento cestisti francesi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Jonathan Jeanne Nazionalità Francia Altezza 218 cm Peso 93 kg Pallacanestro Ruolo Ala grande / centro Squadra Poitiers CarrieraGiovanili Asc Ban E Lot2012-2015 INSEP2015-2016 Le MansSquadre di club 2013-2015 INSEP59 (404)2015-2016 Le Mans6 (8)2016-2017→ Nancy19 (60)2018-...

Ice hockey league in Finland LiigaCurrent season, competition or edition: 2023–24 Liiga seasonFormerlySM-sarja (1933–1975)SM-liiga (1975–2013)SportIce hockeyFounded1975; 49 years ago (1975)First season1975–76CEOMikko PulkkinenMottoSe on totta (It's for real)No. of teams16CountryFinlandMost recentchampion(s)Tappara (13th title) (2023–24)Most titlesTappara (13 titles)TV partner(s)Telia Company, TV5Level on pyramidLevel 1Relegation toMestisInternational cup(s)Champion...

American football player and coach (born 1955) Charlie WeatherbieBiographical detailsBorn (1955-01-17) January 17, 1955 (age 69)Sedan, Kansas, U.S.Playing career1973–1976Oklahoma State1979Ottawa Rough Riders1979–1980Hamilton Tiger-Cats1980Ottawa Rough Riders Position(s)QuarterbackCoaching career (HC unless noted)1977Oklahoma State (GA)1978Enid HS (OK) (assistant)1981–1983Wyoming (QB)1984–1989Air Force (assistant)1990–1991Arkansas (QB)1992–1994Utah State1995–2001Navy2003–2...

Brunnen am Fischhof in Wien, 1737 Brunnen am damaligen Markt Am Hof um 1870 Der Siebenbrunnen in Margareten, Anfang 20. Jahrhundert Die öffentlichen Brunnen in Wien dienten schon seit der Römerzeit der Wasserversorgung des Gemeinwesens und der Brandbekämpfung in der heutigen österreichischen Bundeshauptstadt Wien. Im Lauf der Zeit erhielten die Brunnen zunehmend eine repräsentative Funktion und wurden entsprechend aufwändig gestaltet. Inhaltsverzeichnis 1 Geschichte 2 Brunnen im öffen...

Geostationary communications satellite 0°00′N 120°04′W / 0°N 120.07°W / 0; -120.07 Intelsat 603Astronauts working on Intelsat 603 during STS-49Mission typeCommunicationOperatorIntelsatCOSPAR ID1990-021A[1]SATCAT no.20523[1]Mission duration13 years (planned)23 years (achieved) Spacecraft propertiesBusHS-389ManufacturerHughes[2]Launch mass4,215 kilograms (9,292 lb)[2] Start of missionLaunch date14 March 1990, 11:52:31 (19...

Salim AhamedSalim AhamedLahirP. P. Salim Ahamed1 Oktober 1970 (umur 53)Mattannur, Kannur, Kerala, IndiaPekerjaanSutradara film, penulis naskah, produser filmTahun aktif2011 – sekarang Ini adalah nama India; nama Ahamed merupakan patronimik, bukan nama keluarga, dan tokoh ini dipanggil menggunakan nama depannya, Salim. Salim Ahamed adalah seorang sutradara film, penulis latar dan produser India. Setelah bekerja dalam waktu yang lama sebagai konsultan perjalanan, ia bergabung sebag...

إيونيةمعلومات عامةالثقافة اليونان القديمة تقع في منطقة تضاريس الأناضول الإحداثيات 38°12′N 27°30′E / 38.2°N 27.5°E / 38.2; 27.5 تعديل - تعديل مصدري - تعديل ويكي بيانات جزء من شبه جزيرة الأناضول، والتي توضح وضع إيونية القديمة. إيونية هي مدينة إغريقية قديمة تقع على الساحل الغر�...

Pier and amusement park in New Jersey This article is about the pier in Atlantic City. For the musical named after the pier, see Steel Pier (musical). Steel PierSteel Pier c. 1915Location1000 Boardwalk, Atlantic City, New Jersey, U.S.Coordinates39°21′27″N 74°25′08″W / 39.3575°N 74.4190°W / 39.3575; -74.4190StatusOperatingOpenedJune 18, 1898OwnerSteel Pier Associates, LLCOperating seasonApril through October (Observation Wheel open March–December)Att...

Contract between the King of Poland and Polish nobility Part of a series on the politics and government of PolandConstitutions and major statutes of Poland Neminem captivabimus acts (1430) Nihil novi act (1505) Henrician Articles (1573) Constitution of 3 May 1791 Warsaw Duchy Constitution (1807) Kingdom of Poland (1815) Organic Statute (1832) Small Constitution (1919) March Constitution (1921) August Novelization (1926) April Constitution (1935) Small Constitution (1947) People's Republic (19...

جزيرة سانتا روزا (كاليفورنيا) معلومات جغرافية الإحداثيات 33°57′00″N 120°06′03″W / 33.950003°N 120.1009639°W / 33.950003; -120.1009639 [1] [2] الأرخبيل جزر القناة في كاليفورنيا المسطح المائي المحيط الهادئ المساحة 83.12 ميل مربع الطول 26 كيلومتر العرض 17 كيلومتر ...

Vous lisez un « bon article » labellisé en 2020. Pour les articles homonymes, voir Halimi. Gisèle Halimi Gisèle Halimi en 2008. Fonctions Ambassadrice de la France à l'UNESCO 13 avril 1985 – 1er septembre 1986(1 an, 4 mois et 19 jours) Directeur général Amadou-Mahtar M'Bow Prédécesseur Jacqueline Baudrier Successeur Marie-Claude Cabana Députée française 2 juillet 1981 – 9 septembre 1984(3 ans, 2 mois et 7 jours) Élection 21 juin 1981 ...

التكنولوجيا الفائقة أو هاي تيك (بالأنجليزية: High-tech architecture) أو (Late Modernism) طراز معماري ظهر في سبعينيات القرن العشرين والذي تضمن عناصر الصناعة والتكنلوجيا الفائقة الحديثة في مجال تصميم المباني. وجدت العمارة فائقة التكنولوجيا بعد مرحلة تجديد الحداثة، امتدادا للأفكار السابقة، �...

Mathematical treatise by Bhāskara II For other uses, see Leelavathi (disambiguation). Līlāvatī is a treatise by Indian mathematician Bhāskara II on mathematics, written in 1150 AD. It is the first volume of his main work, the Siddhānta Shiromani,[1] alongside the Bijaganita, the Grahaganita and the Golādhyāya.[2] A problem from the Lilavati by Bhaskaracharya. Written in the 12th century. This appeared on page 18 of The Mathematical Mystery Tour by UNESCO in 1989. Name ...

Part of a series on the History of Cuba Governorate of Cuba (1511–1519) Viceroyalty of New Spain (1535–1821) Siege of Havana (1762) Captaincy General of Cuba (1607–1898) Lopez Expedition (1850–1851) Ten Years' War (1868–1878) Little War (1879–1880) Cuban War of Independence (1895–1898) Treaty of Paris (1898) US Military Government (1898–1902) Platt Amendment (1901) Republic of Cuba (1902–1959) Cuban Pacification (1906–1909) Negro Rebellion (1912) Sugar Intervention (1917�...

Ancient religious monument in Rome, Italy For other uses, see Sanctuary of Hercules Victor (Tivoli). Temple of Hercules VictorThe Temple of Hercules Victor, in the Forum BoariumTemple of Hercules VictorShown within Augustan RomeClick on the map for a fullscreen viewCoordinates41°53′19″N 12°28′51″E / 41.8887°N 12.4808°E / 41.8887; 12.4808 The Temple of Hercules Victor (Italian: Tempio di Ercole Vincitore) or Hercules Olivarius (Latin for Hercules the Olive-B...

TNPO2 معرفات أسماء بديلة TNPO2, IPO3, KPNB2B, TRN2, transportin 2 معرفات خارجية الوراثة المندلية البشرية عبر الإنترنت 603002 MGI: MGI:2384849 HomoloGene: 8381 GeneCards: 30000 علم الوجود الجيني وظائف جزيئية • GO:0001948، GO:0016582 ربط بروتيني• nuclear localization sequence binding مكونات خلوية • غلاف نووي• nuclear periphery• نواة• سيتوبلازم عم...


