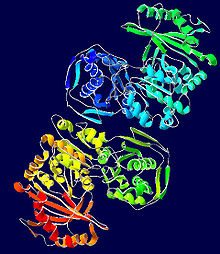
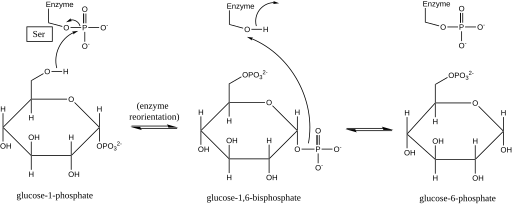
Phosphoglucomutase
| |||||||||||||||||||||||||||||||||||||
Read other articles:

Aequorea Aequorea victoria (jeli kristal) dengan dua amphipodaTaksonomiKerajaanAnimaliaFilumCnidariaKelasHydrozoaOrdoLeptothecataFamiliAequoreidaeGenusAequorea Péron dan Lesueur, 1809 lbs Aequorea adalah sebuah genus dari ubur-ubur Hydrozoa pelagik dari familia Aequoreidae.[1] Spesies Berikut adalah spesies dalam genus Aequorea: Aequorea africana Millard, 1966 Aequorea albida L. Agassiz, 1862 Aequorea atrikeelis Lin, Xu, Huang & Wang, 2009 Aequorea australis Uchida, 1947 Aequore...

Halaman ini berisi artikel tentang istana di Damaskus. Untuk istana di Hamat yang dibangun oleh klien yang sama, lihat Istana Azm (Hamat). Istana Azmقصر العظمNama lainQasr al-AzmInformasi umumJenisIstana, MuseumGaya arsitekturOttomanLokasiDamascus, SyriaAlamatAl-Buzuriyah SouqRampung1750Tanggal renovasi1945-1961KlienAs'ad Pasha al-AzmData teknisJumlah lantai2Tim renovasiPenghargaanPenghargaan Aga Khan untuk Arsitektur Istana Azm (Arab: قصر العظمcode: ar is deprecated ) adalah ...

This article is about the area of Edinburgh. For the cricket club of the same name, see The Grange Club. Human settlement in ScotlandThe GrangeVilla in The GrangeThe GrangeLocation within the City of Edinburgh council areaShow map of the City of Edinburgh council areaThe GrangeLocation within ScotlandShow map of ScotlandOS grid referenceNT260716Council areaCity of EdinburghLieutenancy areaEdinburghCountryScotlandSovereign stateUnited KingdomPost townEDINBURGHPostcode&...

Chronologies Louis XV chassant le cerf, Jean-Baptiste Oudry, 1730.Données clés 1727 1728 1729 1730 1731 1732 1733Décennies :1700 1710 1720 1730 1740 1750 1760Siècles :XVIe XVIIe XVIIIe XIXe XXeMillénaires :-Ier Ier IIe IIIe Chronologies thématiques Art Architecture, Arts plastiques (Dessin, Gravure, Peinture et Sculpture), Littérature, Musique classique et Théâtre Ingénierie (), Architecture et () Politiqu...

Mata ayam Klasifikasi ilmiah Kerajaan: Plantae Divisi: Magnoliophyta Kelas: Magnoliopsida Ordo: Arecales Famili: Myrsinaceae Genus: Ardisia Spesies: A. crispa Nama binomial Ardisia crispa Mata ayam atau juga dikenali sebagai mata pelanduk adalah tumbuhan renek yang mempunyai daun yang memanjang dan bergeligih. Tumbuhan ini merupakan sejenis tumbuhan tropikal. Nama sainsnya Ardisia crispa. Pokok Mata Pelanduk/Mata Ayam. Pengidentifikasi taksonArdisia crispa Wikidata: Q11294922 Wikispecie...

Untuk kitab Alkitab, lihat Kitab Ester. Untuk istilah gugus senyawa kimia, lihat Ester (kimia). Untuk kegunaan lain, lihat Ester (disambiguasi). Ester (bahasa Ibrani: אֶסְתֵּר, Modern Ester Tiberias ʼEstēr; Inggris: Esther), lahir dengan nama Hadasa (Inggris: Hadassah), anak Abihail, seorang Yahudi yang tinggal di Persia. Ia yang menjadi tokoh utama dalam Kitab Ester, bagian dari Alkitab Ibrani dan Perjanjian Lama di Alkitab Kristen. Dicatat bahwa ia dipilih menjad...

Questa voce o sezione sull'argomento governi non cita le fonti necessarie o quelle presenti sono insufficienti. Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Governo di Rudinì IV Stato Italia Presidente del ConsiglioAntonio di Rudinì(Destra storica) CoalizioneDestra storica LegislaturaXX Giuramento14 dicembre 1897 Dimissioni28 maggio 1898 Governo successivoDi Rudinì V1º giugno 1898 Di Rudinì III Di Rudinì V...

Questa voce sull'argomento popoli antichi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Territori slavi verso il 1150 Gli Evelli (Heveller in lingua tedesca) furono una popolazione di origine slava che visse nel medio bacino del fiume Havel e che apparteneva al gruppo dei Venedi (slavi dell'Elba e del Mar Baltico). La loro denominazione propria era Stodorjane. Il nome alto-tedesco moderno Heveller (da cui l'italiano Evelli) proviene da una forma di ...

Very thin gold used in art Goldleaf redirects here. For other uses, see Gold leaf (disambiguation). This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Gold leaf – news · newspapers · books · scholar · JSTOR (December 2009) (Learn how and when to remove this template message) A gold nugget of 5 mm (0.2 in) in di...

Defunct football club in Calais, France For the active football club based in Calais, see RC Calais. Football clubCRUFCFull nameCalais Racing Union Football ClubNickname(s)Les Sangs et Ors (The Blood(-Reds) and Golds) Les Canaris (The Canaries)Founded1974Dissolved2017GroundStade de l'ÉpopéeCapacity12,432 Home colours Away colours Calais Racing Union FC (Calais RUFC) was a French football club based in Calais, France. Calais RUFC was founded in 1974 after a merger of two local clubs and, as ...

この記事は検証可能な参考文献や出典が全く示されていないか、不十分です。出典を追加して記事の信頼性向上にご協力ください。(このテンプレートの使い方)出典検索?: コルク – ニュース · 書籍 · スカラー · CiNii · J-STAGE · NDL · dlib.jp · ジャパンサーチ · TWL(2017年4月) コルクを打ち抜いて作った瓶の栓 コルク(木栓、�...

2010 single by Nelly Just a DreamSingle by Nellyfrom the album 5.0 ReleasedAugust 16, 2010 (2010-08-16)Recorded2010StudioThe Hit FactoryPlayland Playhouse (Miami, Florida)GenrePop rapR&BLength3:57LabelDerrtyUniversal MotownSongwriter(s)Cornell Haynes Jr.James SchefferRichard Butler, Jr.Frank RomanoProducer(s)Jim JonsinRico LoveNelly singles chronology Stepped on My J'z (2008) Just a Dream (2010) Move That Body (2010) Music videoJust a Dream on YouTube Just a Dream is a song...

African-American author and activist Frances HarperBornFrances Ellen WatkinsSeptember 24, 1825Baltimore, MarylandDiedFebruary 22, 1911(1911-02-22) (aged 85)Philadelphia, PennsylvaniaGenrePoetry, short story, essaysNotable worksIola Leroy (1892) Sketches of Southern Life (1872)SpouseFenton Harper (m. 1860)ChildrenMary Frances Harper (1862–1908)Frances Ellen Watkins Harper (September 24, 1825 – February 22, 1911) was an American abolitionist, suffragist, poet, temperance activist, teac...

جامعة لفوف الطبية الوطنية بأسم دانيلا هاليتسكوهو Львівський національний медичний університет імені Данила Галицького شعار جامعة لفوف الطبية الوطنية باسم دانيلا هاليتسكوهوЛНМУ معلومات التأسيس 1784 النوع جامعة حكومية الموقع الجغرافي إحداثيات 49°50′02″N 24°03′12″E / ...

German archaeologist (1806–1859) Ludwig RossLudwig Ross, photographed in later lifeBorn(1806-07-22)22 July 1806Bornhöved, Holstein, DenmarkDied6 August 1859(1859-08-06) (aged 53)Halle an der SaaleOccupationArchaeologistKnown forEphor General of Antiquities of Greece; restoration of the Temple of Athena Nike.TitleEphor General (1834–1836)Spouse Emma Schwetschke (m. 1847)RelativesCharles Ross (brother)Academic backgroundEducationChristian-Albrechts...

Gambrinus liga 2000/01Datos generalesSede República Checa República ChecaFecha 28 de julio del 200025 de mayo de 2001Edición 8Organizador Federación de Fútbol de la República ChecaPalmarésPrimero AC Sparta PragaSegundo SK Slavia PragaTercero SK Sigma OlomoucDatos estadísticosParticipantes 16Partidos 240Partidos jugados 240Máximo goleador Vítězslav Tuma (15) Intercambio de plazas Ascenso(s): SK Hradec Králové SFC Opava Descenso(s): SK České Budějovice FC Viktoria PlzeňCro...

هذه المقالة تحتاج للمزيد من الوصلات للمقالات الأخرى للمساعدة في ترابط مقالات الموسوعة. فضلًا ساعد في تحسين هذه المقالة بإضافة وصلات إلى المقالات المتعلقة بها الموجودة في النص الحالي. (فبراير 2023) بطولة إفريقيا للجودو 2004معلومات عامةموسم لـ بطولة إفريقيا للجودو الاسم المختص...
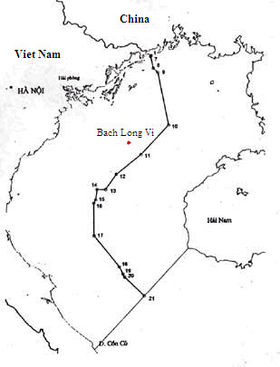
此条目页的主題是北部湾上的一个岛屿。关于该岛所属的越南海防市下辖的县份,請見「白龙尾县」。 白龙尾岛Bạch Long Vĩ白龙尾岛卫星照片白龙尾岛白龙尾岛的位置地理位置北部湾坐标20°08′41″N 107°42′51″E / 20.14472°N 107.71417°E / 20.14472; 107.71417 (Thổ Chu Island)面積3.045平方公里(1.176平方英里)管轄 越南市海防市县白龙尾县人口统计人...

Method of divination that interprets markings on the ground Geomancer redirects here. For other uses, see Geomancer (disambiguation). This article is about the African and European divination technique. For the Chinese art of aesthetics, see Feng shui. For the Chinese philosophical tradition, see Wuxing (Chinese philosophy). This article may be in need of reorganization to comply with Wikipedia's layout guidelines. Please help by editing the article to make improvements to the overall structu...

German publisher, founder of Kurt Wolff Verlag For the World War I flying ace, see Kurt Wolff (aviator). Some books published by Kurt Wolff Kurt Wolff (3 March 1887 – 21 October 1963) was a German publisher, editor, writer, and journalist. Wolff was born in Bonn, Rhenish Prussia; his mother came from a Jewish-German family.[1] He married Elisabeth Karoline Clara Merck (1890–1970), of the Darmstadt pharmaceuticals firm, in 1909. Together with Ernst Rowohlt, Wolff began to work ...