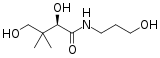
Panthenol
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

العلاقات الدومينيكية السنغافورية دومينيكا سنغافورة دومينيكا سنغافورة تعديل مصدري - تعديل العلاقات الدومينيكية السنغافورية هي العلاقات الثنائية التي تجمع بين دومينيكا وسنغافورة.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية �...

Georgian coder This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (May 2017) Faramarz Fekri from the Georgia Institute of Technology, Atlanta was named Fellow of the Institute of Electrical and Electronics Engineers (IEEE) in 2016[1] for contributions to coding theory and its applications. References ^ 2016 elevated fellow (PDF). IEEE Fellows Directory. Archived from the origina...

Untuk kegunaan lain, lihat Semen (disambiguasi). Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Semen, Paron, Ngawi – berita · surat kabar · buku · cendekiawan · JSTOR SemenDesaNegara IndonesiaProvinsiJawa TimurKabupatenNgawiKecamatanParonKode p...

Questa voce sull'argomento festività è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Coniglietti pasquali ricamati su una tovaglia Coniglietti pasquali di cioccolato Il coniglio pasquale (in inglese Easter Bunny, lett. coniglietto di Pasqua; in tedesco Osterhase, lett. lepre di Pasqua), conosciuto anche come coniglio di primavera, coniglietto pasquale o coniglio di Pasqua nei paesi di lingua...

Max S. NordauLahirSimon Maximilian (Simcha) Südfeld(1849-07-29)29 Juli 1849Pest, Austria-Hungaria (sekarang Budapest, Hungaria)Meninggal23 Januari 1923(1923-01-23) (umur 73)Paris, PrancisPekerjaanDokter, penulis, dan kritikus sosialDikenal atasSalah satu pendiri Organisasi Zionis SeduniaKarya terkenalDegenerasi Max Simon Nordau (nama lahir Simon Maximilian Südfeld; 29 Juli 1849 – 23 Januari 1923) adalah seorang pemimpin Zionis, dokter, penulis dan kritikus sosial.[...

Dutch politician and farmer (born 1979) In this Dutch name, the birth surname is Monaster and the marital name is Vedder. Eline VedderVedder (center) at a 2021 farmers' protestMember of the House of RepresentativesIncumbentAssumed office 10 May 2023Preceded byJaco GeurtsMember of the Provincial Council of DrentheIn office28 March 2019 – 30 May 2023Succeeded bySonja Hilgenga-van DamMember of the Municipal Council of De WoldenIn office29 March 2018 – 25 April 2...

Willsboro redirects here. For similarly named places, see Willsborough (disambiguation). Town in New York, United StatesWillsboro, New YorkTownPaine Memorial Library, built in 1930Location in Essex County and the state of New YorkCoordinates: 44°22′29″N 73°24′24″W / 44.37472°N 73.40667°W / 44.37472; -73.40667CountryUnited StatesStateNew YorkCountyEssexGovernment • TypeTown Council • Town SupervisorShaun Gillilland (R) • ...

Former college basketball player Kye AllumsPersonal informationBornOctober 23, 1989 (1989-10-23) (age 34)NationalityAmericanCareer informationHigh schoolCentennial High SchoolCollegeGeorge Washington Kye Allums (born October 23, 1989) is a former college basketball player for the George Washington University women's team who in 2010 came out as a trans man, becoming the first openly transgender NCAA Division I college athlete.[1][2][3][4][5] A...

Peta lokasi Munisipalitas Slagelse Munisipalitas Slagelse adalah munisipalitas (Denmark: kommune) di Region Sjælland di Denmark. Munisipalitas Slagelse memiliki luas sebesar 571 km² dan memiliki populasi sebesar 77.457 jiwa. Referensi Municipal statistics: NetBorger Kommunefakta Diarsipkan 2007-08-12 di Wayback Machine., delivered from KMD aka Kommunedata (Municipal Data) Municipal merges and neighbors: Eniro new municipalities map Diarsipkan 2007-10-11 di Wayback Machine. lbsPemukiman...

American poet laureate, writer, and librarian (1841–1928) Ina Coolbrith in the 1880s Ina Donna Coolbrith (born Josephine Donna Smith; March 10, 1841 – February 29, 1928) was an American poet, writer, librarian, and a prominent figure in the San Francisco Bay Area literary community. Called the Sweet Singer of California,[1] she was the first California Poet Laureate and the first poet laureate of any American state.[2] Coolbrith, born the niece of the Church of Jesus Chris...
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)&#...

烏克蘭總理Прем'єр-міністр України烏克蘭國徽現任杰尼斯·什米加尔自2020年3月4日任命者烏克蘭總統任期總統任命首任維托爾德·福金设立1991年11月后继职位無网站www.kmu.gov.ua/control/en/(英文) 乌克兰 乌克兰政府与政治系列条目 宪法 政府 总统 弗拉基米尔·泽连斯基 總統辦公室 国家安全与国防事务委员会 总统代表(英语:Representatives of the President of Ukraine) 总...

本條目存在以下問題,請協助改善本條目或在討論頁針對議題發表看法。 此條目需要編修,以確保文法、用詞、语气、格式、標點等使用恰当。 (2013年8月6日)請按照校對指引,幫助编辑這個條目。(幫助、討論) 此條目剧情、虛構用語或人物介紹过长过细,需清理无关故事主轴的细节、用語和角色介紹。 (2020年10月6日)劇情、用語和人物介紹都只是用於了解故事主軸,輔助�...

مرقس السابع معلومات شخصية مكان الميلاد أوكسيرينخوس الوفاة مايو 18, 1769إيالة مصر مكان الدفن كنيسة أبو سيفين الإقامة كنيسة العذراء المغيثة مواطنة مصر الحياة العملية المهنة قسيس اللغة الأم القبطية اللغات اللهجة المصرية تعديل مصدري - تعديل قداسة البابا ...

Congresso di Lubiana Temaazioni da intraprendere dopo i moti rivoluzionari del 1820 Apertura26 gennaio - 12 maggio 1821 Stato Impero austriaco LocalitàLubiana Il congresso di Lubiana fu un incontro delle forze della Restaurazione, avvenuto nel gennaio 1821, in seguito ai moti rivoluzionari scoppiati l'anno precedente in Spagna (1º gennaio), nel regno delle Due Sicilie (1º luglio) e in Portogallo (24 agosto). Metternich propugnò l'intervento armato per il mantenimento degli equilibri ...

Здание бывшей Главной почтовой дирекции Ганновера Гла́вная почто́вая дире́кция (нем. Oberpostdirektion; OPD; ГПД) — административная единица и орган почтового управления среднего звена в Германии. Впервые дирекции были созданы в 1850 году в Пруссии. После Второй мировой войны ...

Global contract research organization PPD, Inc.PPD Headquarters in Wilmington, NCCompany typeSubsidiaryIndustryContract research organizationsPharmaceuticalBiotechnologyFounded1985HeadquartersWaltham, Massachusetts, United StatesKey peopleSee Senior Leadership Team page of the PPD Website[1]ProductsContract clinical research for pharmaceutical, biotechnology, medical device, academic and government organizations; services include drug development, laboratory and lifecycle management.R...

Canadian technology company This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. (October 2022) (Learn how and when to remove this message) This article contains content that is written like an advertisement. Please help impr...

Animated Disney film For the Disney franchise, see Snow White (franchise). Snow White and the Seven DwarfsTheatrical release poster by Gustaf TenggrenDirected by David Hand Perce Pearce William Cottrell Larry Morey Wilfred Jackson Ben Sharpsteen Story by Ted Sears Richard Creedon Otto Englander Dick Rickard Earl Hurd Merrill De Maris Dorothy Ann Blank Webb Smith Based onSnow Whiteby the Brothers GrimmProduced byWalt DisneyMusic by Frank Churchill Leigh Harline Paul Smith ProductioncompanyWalt...

العلاقات المالديفية التشيلية جزر المالديف تشيلي المالديف تشيلي تعديل مصدري - تعديل العلاقات المالديفية التشيلية هي العلاقات الثنائية التي تجمع بين المالديف وتشيلي.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه ا�...