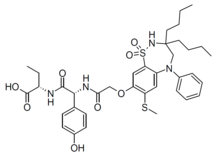
Odevixibat
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Artikel ini perlu diwikifikasi agar memenuhi standar kualitas Wikipedia. Anda dapat memberikan bantuan berupa penambahan pranala dalam, atau dengan merapikan tata letak dari artikel ini. Untuk keterangan lebih lanjut, klik [tampil] di bagian kanan. Mengganti markah HTML dengan markah wiki bila dimungkinkan. Tambahkan pranala wiki. Bila dirasa perlu, buatlah pautan ke artikel wiki lainnya dengan cara menambahkan [[ dan ]] pada kata yang bersangkutan (lihat WP:LINK untuk keterangan lebih lanjut...

Study of the Bible Bible reading redirects here. For reading in church, see Lection. For the academic field, see biblical studies. Part of a series on theBible Canons and books Tanakh Torah Nevi'im Ketuvim Old Testament (OT) New Testament (NT) Deuterocanon Antilegomena Chapters and verses Apocrypha Jewish OT NT Authorship and development Authorship Dating Hebrew canon Old Testament canon New Testament canon Composition of the Torah Mosaic authorship Pauline epistles Petrine epistles Johannine...
Mass media in Pittsburgh Pittsburgh is home to the first commercial radio station in the United States, KDKA 1020AM, the first community-sponsored television station in the United States, WQED 13, the first networked television station and the first station in the country to broadcast 24 hours a day, 7 days a week, KDKA 2, and the first newspaper published west of the Allegheny Mountains, the Pittsburgh Post-Gazette. History Until 2016, Pittsburgh was one of the few mid-sized metropolitan are...

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (مارس 2016) مجتمعات المساواة هي مجموعات الأشخاص الذين اختاروا الحياة معًا، بحيث تكون المساواة واحدة من القيم الرئيسية...

Spaceflight-related events of 1995 1995 in spaceflightSpace Shuttle Atlantis launches on STS-71, the first Shuttle-Mir mission to dock with MirOrbital launchesFirst11 JanuaryLast24 DecemberTotal80Successes72Failures5Partial failures3Catalogued74National firstsSatellite Czech Republic (post Czechoslovakian) Ukraine (post Soviet)RocketsMaiden flightsAthena IConestogaDelta II 7920Long March 1DVolnaShavit 1RetirementsAtlas E/FConestogaLong March 2EMu-3SIISoyuz-U2Crewed flightsOrbital9To...

Peringatan Partai Komunis Tiongkok ke-100Bagian dari Dua CentenariLogo resmi acara tersebut, ditampilkan di Distrik Tianxin, Changsha, Hunan, Tiongkok.Nama asli 中国共产党成立100周年庆祝活动Tanggal01 Juli 2021 (2021-07-01)LokasiJalan Chang'an, Lapangan Tiananmen, Beijing, TiongkokKoordinat39°54′26.4″N 116°23′27.9″E / 39.907333°N 116.391083°E / 39.907333; 116.391083Koordinat: 39°54′26.4″N 116°23′27.9″E / 39.90...

Sungai AsahanAek TobaAek AsahanSei AsahanSebuah jembatan yang melintang di daerah hulu sungai Asahan sekitar Porsea, Toba (1929).LokasiNegaraIndonesiaProvinsiSumatera UtaraCiri-ciri fisikHulu sungaiDanau Toba - elevasi950 m (3.120 ft) Muara sungaiSelat Malaka - lokasiBagan Asahan - koordinat3°01′53″N 99°51′47″E / 3.03133°N 99.86306°E / 3.03133; 99.86306Koordinat: 3°01′53″N 99°51′47″E / 3.0313...

System on a chip (SoC) designed by Apple Inc. Apple A16 BionicGeneral informationLaunchedSeptember 7, 2022Marketed byApple Inc.Designed byApple Inc.Common manufacturer(s)TSMCProduct codeAPL1W10[1]PerformanceMax. CPU clock rate2020 MHz to 3460 MHzCacheL2 cache16 MB (performance cores) 4 MB (efficient cores)Last level cache24 MBArchitecture and classificationApplicationMobileTechnology node4 nm N4PMicroarchitectureEverestSawtoothInstructionsARMv8.6-APhysica...

Ziad Jaziri Nazionalità Tunisia Altezza 171 cm Calcio Ruolo Attaccante Termine carriera 2008 Carriera Squadre di club1 1999-2003 Étoile du Sahel87 (47)2003-2005 Gaziantepspor49 (17)2005-2007 Troyes44 (6)2007-2008 Kuwait SC20 (8) Nazionale 1999-2008 Tunisia64 (14) Palmarès Coppa d'Africa Oro Tunisia 2004 1 I due numeri indicano le presenze e le reti segnate, per le sole partite di campionato.Il simbolo → indica un trasferimento in prestito. Modifica...

American television soap opera (1954–1974) This section is about the American soap opera. For the musical act Lauren Hoffman & the Secret Storm, see Lauren Hoffman. The Secret StormTitle card for The Secret Storm, 1954Created byRoy WinsorStarringPeter HobbsJada RowlandMarla AdamsCountry of originUnited StatesNo. of seasons20No. of episodes5,195ProductionRunning time15 minutes (1954–1962, approx. 11–12 minutes minus ads)30 minutes (1962–1974, approx. 22 minutes minus ads)Original r...

The St. Louis flag features two rivers merging in a confluence of French heritage signified by a fleur-de-lis. The Iconography of St. Louis, Missouri is strongly informed by the city's French and German heritages, physical features, and place in American history. Mound City Monks Mound is one of the few remaining mounds in the St. Louis region. Long before Europeans settled in St. Louis, the Cahokia lived throughout the area and constructed many mounds. Though history and population growth wo...

此條目之中立性有争议。其內容、語調可能帶有明顯的個人觀點或地方色彩。 (2011年6月)加上此模板的編輯者需在討論頁說明此文中立性有爭議的原因,以便讓各編輯者討論和改善。在編輯之前請務必察看讨论页。 格奥尔基·季米特洛夫保加利亚共产党中央委员会总书记任期1948年8月—1949年7月2日前任自己(第一书记)继任维尔科·契尔文科夫保加利亚共产党中央委员会第一�...

Un libro in antico slavo ecclesiastico mostra il simbolo atanasiano. Černihiv, Museo. Il simbolo atanasiano (Quicumque vult) è un simbolo della fede che prende questo nome perché attribuito dalla tradizione cristiana al vescovo Atanasio di Alessandria (295-373). È significativo soprattutto per la dottrina trinitaria, che esso esprime in maniera forte per combattere l'arianesimo. Nella liturgia della Chiesa occidentale era recitato nell'ufficio divino domenicale di prima.[1] Nel ri...

Greek dish This article is about the Greek dish. For the moth genus, see Gyros (moth). For other uses, see Gyro (disambiguation). GyrosGyros in Greece, with meat, onions, tomato, lettuce, fried potatoes, and tzatziki rolled in a pitaAlternative namesGyro[1]TypeMeat wrapCourseMain coursePlace of originGreeceServing temperatureHotMain ingredientsMeat: pork, chicken, beef, or lamb Media: Gyros Gyros, sometimes anglicized as a gyro[2][3][4] (/ˈjɪəroʊ,...

Game involving throwing flying discs at the opposing team Guts or disc guts (sometimes guts Frisbee in reference to the trademarked brand name) is a disc game inspired by dodgeball, involving teams throwing a flying disc (rather than balls) at members of the opposing team. Game play One to five team members stand in a line facing the opposing team across the court, with the two teams lined up parallel to each other. Which team begins play is determined by flipping the disc, an action similar ...

Politecnico di Milano La sede di Milano Leonardo UbicazioneStato Italia CittàMilano Altre sediComo, Cremona, Lecco, Mantova, Piacenza Dati generaliSoprannomePoliMI Fondazione29 novembre 1863 FondatoreFrancesco Brioschi TipoPolitecnico statale ScuoleArchitettura Urbanistica Ingegneria delle Costruzioni (AUIC), Design (DESIGN), ingegneria industriale e dell'informazione (3I), Ingegneria Civile Ambientale e Territoriale (ICAT) Dipartimenti12 RettoreDonatella Sciuto[3] Dir. ge...

← січень → Пн Вт Ср Чт Пт Сб Нд 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 2024 рік 20 січня — 20-й день року в григоріанському календарі. До кінця року залишається 345 днів (346 днів — у високосні роки), собор св. Йоана Хрестителя. Цей день в історії: 19 с...

ويكيبيديا اللكسمبورغيةالشعارمعلومات عامةموقع الويب lb.wikipedia.org (اللوكسمبورغية) تجاري؟ لانوع الموقع موسوعة حرةالتأسيس 21 يوليو 2004 الجوانب التقنيةاللغة اللكسمبورغيةترخيص المحتوى رخصة المشاع الإبداعي الملزِمة بالنسب لمؤلِّف العمل وبالترخيص بالمثل غير القابلة للإلغاء 3.0 — �...

建議将此條目或章節併入TVB星河頻道重播電視劇列表。(討論) 此條目没有列出任何参考或来源。 (2019年1月1日)維基百科所有的內容都應該可供查證。请协助補充可靠来源以改善这篇条目。无法查证的內容可能會因為異議提出而被移除。 本列表列出香港TVB星河頻道重播的劇集。 首映年份 電視劇集名稱 集數 2012年翡翠台劇集 愛·回家_(第一輯)Come Home Love 804 首映年份 電視劇�...

Kubok Ukraïny 2010-2011Кубок України Competizione Kubok Ukraïny Sport Calcio Edizione 20ª Date dal 27 luglio 2010al 25 maggio 2011 Luogo Ucraina Partecipanti 53 Risultati Vincitore Šachtar(7º titolo) Secondo Dinamo Kiev Semi-finalisti Arsenal KievDnipro Statistiche Miglior marcatore Andriy Oliynyk (5) Incontri disputati 51 Gol segnati 139 (2,73 per incontro) Lo Shakhtar vincitore della manifestazione Cronologia della competizione 2009-2010 2011-2012 M...