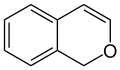
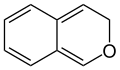
Benzopyran
| |||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Cari artikel bahasa Cari berdasarkan kode ISO 639 (Uji coba) Kolom pencarian ini hanya didukung oleh beberapa antarmuka Halaman bahasa acak Bahasa Jasz yasz, jász, Yasz Dituturkan diHungariaEtnisSuku JaszKepunahanabad ke-15 ? Rumpun bahasaIndo-Eropa Indo-IranIranIran TimurOssetia ?Jasz Kode bahasaISO 639-3yscLINGUIST ListyscGlottologjass1238[1] Status konservasi Punah EXSingkatan dari Extinct (Punah)Terancam CRSingkatan dari Critically endangered (Terancam ...

Carl L. Blume (1847). Karl Ludwig, Ritter von Blume (29 Juni 1789 – 3 Februari 1862) adalah botaniwan Jerman-Belanda. Kadang-kadang namanya ditulis dengan versi Belanda Karel Lodewijk Blume. Kata Ritter adalah gelar kebangsawanan yang dapat disamakan dengan Knight dalam tradisi Anglo-Sakson. Walaupun berkebangsaan Jerman, hampir seluruh karier ilmiahnya dikerjakan di Belanda dan Hindia Belanda (sekarang Indonesia). Puncak kariernya adalah ketika ia menjadi direktur Rijksherbar...

Lukisan Bajo Pivljanin oleh oleh pelukis Serbia Aksentije Marodić (1838–1909). Bercerita tentang Bajo Pavljn yang sedang membunuh seorang Turki (1878). Bajo Pivljanin dikenal sebagai komandan bandit yang memimpin wilayah Utsmani Herzegovina dan Dalmatis.[1] Pivljanin lahir di Piva ketika masa kekaisaran Ottoman pada tahun 1630,[1] dengan nama asli Dragojlo Nikolić. Semasa muda Ia merasa bahwa kekaisaran Ottoman tidak adil kepada rakyatnya, hingga akhirnya Pivljanin memutus...

American filmThe BacchaeDionysus (Richard Werner) in The Bacchae, directed by Brad Mays, 2000.Directed byBrad MaysWritten byBrad Mays, adapted from EuripidesProduced byLorenda Starfelt, John MorrisseyStarringRichard Werner, Jonathan Klein, Lynn Odell, William Dennis Hunt, Will Shepherd, Ramona Reeves, Elyse Ashton, Kiersten MorganCinematographyJacob PingerEdited byBrad MaysMusic byPeter GirardRunning time88 minutesCountryUnited StatesLanguageEnglish The Bacchae is an independent film adaptati...

BimbisaraBimbisara menyambut BuddhaPendiri Dinasti HaryankaBerkuasaskt. 544 - 492 SM (52 tahun) atau skt. 400 SMPendahuluBhattiyaPenerusAjatashatruInformasi pribadiKelahiran558 SMKematian491 SMAyahBhattiyaPasanganKosala DeviChellanaKshemaAnakAjatasatru, AbhayAgamaJainisme, Buddhisme Bimbisara (skt. 558-491 SM[1][2] atau pada akhir abad ke-5 SM[3] juga dikenal sebagai Srenika di dalam sejarah-sejarah Jain [4][5] merupakan seorang Raja Magadha (bertakhta ...

Election for governor of Maryland, U.S. 1982 Maryland gubernatorial election ← 1978 November 2, 1982 1986 → Nominee Harry Hughes Robert A. Pascal Party Democratic Republican Running mate J. Joseph Curran Jr. Newton Steers Popular vote 705,910 432,826 Percentage 61.97% 38.00% County resultsHughes: 50-60% 60-70% 70-80%Pascal: 50-60% Gover...

1954 American filmYankee Doodle BugsScreenshot of Yankee Doodle Bugs title cardDirected byI. FrelengStory byWarren FosterProduced byEdward Selzer (uncredited)StarringMel BlancBea Benaderet (uncredited)[1]Music byMilt FranklynAnimation byArthur DavisManuel PerezVirgil RossHarry Love (uncredited)Layouts byHawley PrattBackgrounds byIrv WynerColor processTechnicolorProductioncompanyWarner Bros. CartoonsDistributed byWarner Bros.VitaphoneRelease date August 28, 1954 (1954-08...

Chemical compound AzelastineClinical dataTrade namesAstelin, Optivar, Allergodil, others.[1]AHFS/Drugs.comMonographMedlinePlusa603009License data US DailyMed: Azelastine Pregnancycategory AU: B3 Routes ofadministrationEye drops, nasal spray, by mouthATC codeR01AC03 (WHO) R06AX19 (WHO), S01GX07 (WHO)Legal statusLegal status AU: S2 (Pharmacy medicine) UK: POM (Prescription only) US: ℞-only / OTC[2] Pharmacokinetic dat...

County in Sichuan, ChinaLitang County 理塘县 • ལི་ཐང་རྫོང་།CountyLandscape of Litang CountyLitang County (red) in Garzê Prefecture (yellow) and SichuanLitangLocation of the seat in SichuanShow map of SichuanLitangLitang (China)Show map of ChinaCoordinates (Litang County government): 29°59′39″N 100°16′09″E / 29.9942°N 100.2692°E / 29.9942; 100.2692CountryChinaProvinceSichuanAutonomous prefectureGarzêCounty seatTochong (Lita...

Mountain Viewcity(EN) City of Mountain View Mountain View – Veduta LocalizzazioneStato Stati Uniti Stato federato California ConteaSanta Clara AmministrazioneSindacoLucas Ramirez TerritorioCoordinate37°23′34″N 122°02′31″W / 37.392778°N 122.041944°W37.392778; -122.041944 (Mountain View)Coordinate: 37°23′34″N 122°02′31″W / 37.392778°N 122.041944°W37.392778; -122.041944 (Mountain View) Altitudine32 m s.l.m. Superf...
周處除三害The Pig, The Snake and The Pigeon正式版海報基本资料导演黃精甫监制李烈黃江豐動作指導洪昰顥编剧黃精甫主演阮經天袁富華陳以文王淨李李仁謝瓊煖配乐盧律銘林孝親林思妤保卜摄影王金城剪辑黃精甫林雍益制片商一種態度電影股份有限公司片长134分鐘产地 臺灣语言國語粵語台語上映及发行上映日期 2023年10月6日 (2023-10-06)(台灣) 2023年11月2日 (2023-11-02)(香�...

Лейопельмы Северная лейопельма Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКласс:ЗемноводныеПодкла...

Issues of racism and race relations in various countries Part of a series onDiscrimination Forms Institutional Structural Statistical Taste-based Attributes Age Caste Class Dialect Disability Genetic Hair texture Height Language Looks Mental disorder Race / Ethnicity Skin color Scientific racism Rank Sex Sexual orientation Species Size Viewpoint Social Arophobia Acephobia Adultism Anti-albinism Anti-autism Anti-homelessness Anti-drug addicts Anti-intellectualism Anti-intersex Anti-le...
Kurt Gödel (1925 circa) Kurt Friedrich Gödel (Brno, 28 aprile 1906 – Princeton, 14 gennaio 1978) è stato un matematico, logico e filosofo austriaco naturalizzato statunitense, noto soprattutto per i suoi lavori sull'incompletezza delle teorie matematiche. Ritenuto uno dei più grandi logici di tutti i tempi insieme ad Aristotele, Leibniz e Frege,[1] le sue ricerche ebbero un significativo impatto, oltre che sul pensiero matematico e informatico, anche sul pensiero filosofico del ...

Protective scriptures in Buddhism Translations ofParittaEnglishprotection, safeguardSanskritparitranaPaliparittaBurmeseပရိတ်(Parit) (MLCTS: pəjeiʔ)Khmerបរិត្ត (UNGEGN: Paret)Sinhalaපිරිත් (pirit)ThaiปริตรRTGS: paritGlossary of Buddhism Part of a series onBuddhism Glossary Index Outline History Timeline The Buddha Pre-sectarian Buddhism Councils Silk Road transmission of Buddhism Decline in the Indian subcontinent Later Buddhists Buddhist mode...

Iranian-Jewish journalist and Zionist Shmuel Hayyim. First page of Ha-Hayyim newspaper which was published between 1922 and 1925. Shmuel Hayyim (HE: שמואל חיים) (born 1891 - died 1931), also known as Shmuel Eshagh Effendi or Monsieur Hayyim, was a Persian Jewish journalist, leader of the Iranian Jewish community, a Zionist, and a member of the Majlis (Iranian Parliament). Biography Hayyim was born in Kermanshah. He studied in the Alliance Israelite Universelle schools in Kermanshah a...

Wedding Agreement the SeriesGenre Drama Roman PembuatDisney+ HotstarBerdasarkanWedding Agreementoleh Archie HekagerySkenario Archie Hekagery Mia Chuz SutradaraArchie HekageryPemeran Indah Permatasari Refal Hady Susan Sameh Ibrahim Risyad Yoshi Sudarso Lagu pembukaKontras — Figura RenataLagu penutupJawab Cinta — dUAMusik Tya Subiakto Negara asalIndonesiaBahasa asliBahasa IndonesiaJmlh. musim2Jmlh. episode20 (daftar episode)ProduksiProduser eksekutif Mithu Nisar Riza Raza Servia Amrit Dido...

Roschdy ZemRoschdy Zem pada 2017 di acara Globes de Cristal Award.Lahir27 September 1965 (umur 58)Gennevilliers, Hauts-de-Seine, PrancisPekerjaanPemeran, sutradara, penulis naskah, produser Roschdy Zem (lahir 27 September 1965) adalah seorang pemeran dan pembuat film Prancis keturunan Maroko. Ia meraih penghargaan untuk Aktor Terbaik untuk perannya dalam film Days of Glory di Festival Film Cannes 2006.[1] Referensi ^ Festival de Cannes: Days of Glory. festival-cannes.com. Diakse...

努古里亞群島北環礁衛星圖 努古里亞群島(英語:Nuguria)是巴布亞新畿內亞布干维尔自治区的群島,位於新愛爾蘭島東北200公里的太平洋海域,由二個環礁、約50座島嶼組成,總土地面積10平方公里,2000年人口502。 外部連結 Oceandots page on the Islands(页面存档备份,存于互联网档案馆) 这是一篇與巴布亚新几内亚地理相關的小作品。您可以通过编辑或修订扩充其内容。查论�...

سلافيةالإثنية:سلافالتوزيعالجغرافي:في جميع أنحاء وسط وشرق أوروبا وروسياتصنيفات اللغوية:هندية أوروبيةبلطيقية سلافيةسلافيةاللغة البدائية:سلافية أمفروع: سلافية شرقية سلافية جنوبية سلافية غربية أيزو 2-639 / 5:slaالمرصد اللغوي:53= (phylozone)غلوتولوغ:slav1255[1] اللغات السلافية الغربي...