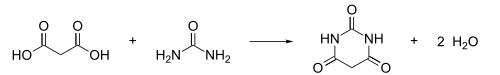
Barbituric acid
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

American television personality, producer, and former model Tyra BanksBanks in 2020BornTyra Lynne Banks (1973-12-04) December 4, 1973 (age 50)Inglewood, California, U.S.Other namesBanXAlma materImmaculate Heart High SchoolOccupations Model television personality producer writer actress businesswoman Years active1991–presentTelevision America's Next Top Model The Tyra Banks Show America's Got Talent FABLife Dancing with the Stars Height5 ft 10 in (1.78 m)&#...

Cet article concerne le groupe. Pour l'album homonyme, voir Queens of the Stone Age (album). Queens of the Stone Age Queens of the Stone Age en concert aux Eurockéennes en 2007Informations générales Autre nom QotSA Pays d'origine États-Unis Genre musical Rock alternatif, stoner rock, rock industriel, art rock, hard rock, metal alternatif[1] Années actives Depuis 1996 Labels Matador Records, MCA, Interscope Records, Roadrunner Records Site officiel www.qotsa.com Composition du groupe...

City in Maine, United States 04240 City in Maine, United StatesLewiston, MaineCityTop: Civil War Memorial Statue, Bates College's Hathorn Hall; Bottom: the Wallace School, Kennedy Park and Lewiston City Hall SealNicknames: The Lew[1]Little Canada[2]Petit Canada[2]Motto(s): Industria (Latin)Industry[3]Interactive map of LewistonLewistonLocation in MaineShow map of MaineLewistonLocation in the United StatesShow map of the United StatesCoordinates: 44°0...
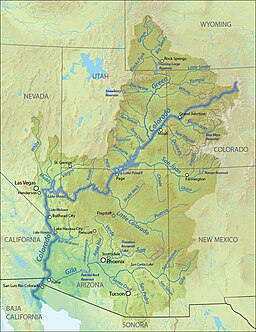
Sungai Colorado Sungai Colorado di Horseshoe Bend, Arizona, beberapa mil di bawah Bendungan Glen Canyon Countries Amerika Serikat, Meksiko Provinsi Colorado, Utah, Arizona, Nevada, California, Baja California, Sonora Anak sungai - kiri Sungai Fraser, Sungai Blue, Sungai Eagle, Sungai Roaring Fork, Sungai Gunnison, Sungai Dolores, Sungai San Juan, Sungai Little Colorado, Sungai Bill Williams, Sungai Gila - kanan Sungai Green, Sungai Dirty Devil, Sungai Escalante, Sungai ...

Horse owned by George Washington Portrait of George Washington Taking the Salute at Trenton by John Faed, featuring Blueskin Blueskin was a gray horse ridden by George Washington. He was one of Washington's two primary mounts during the American Revolutionary War. The horse was a half-Arabian, sired by the stallion Ranger, also known as Lindsay's Arabian, said to have been obtained from the Sultan of Morocco.[1][2] Blueskin was a gift to Washington from Colonel Benjamin Tasker...

'Allegoria della Prudenza'AutoreTiziano Data1565-70 ca. Tecnicaolio su tela Dimensioni76,2×68,6 cm UbicazioneNational Gallery, Londra Allegoria della Prudenza è il titolo dato ad un quadro di Tiziano raffigurante tre teste umane, un vecchio, un uomo maturo ed un giovane, che sovrastano tre teste animali, rispettivamente un lupo, un leone ed un cane. Eseguito a olio su tela tra il 1565 e il 1570, il quadro misura 76,2 per 68,6 cm. È conservato nella National Gallery di Londra. Ind...

この項目には、一部のコンピュータや閲覧ソフトで表示できない文字が含まれています(詳細)。 数字の大字(だいじ)は、漢数字の一種。通常用いる単純な字形の漢数字(小字)の代わりに同じ音の別の漢字を用いるものである。 概要 壱万円日本銀行券(「壱」が大字) 弐千円日本銀行券(「弐」が大字) 漢数字には「一」「二」「三」と続く小字と、「壱」「�...

American lawyer and politician For the American singer-songwriter, see Jim Lord (singer-songwriter). Jim Lord23rd Treasurer of MinnesotaIn officeJanuary 6, 1975 – January 3, 1983GovernorWendell R. AndersonPreceded byVal BjornsonSucceeded byRobert W. Mattson Jr.Member of the Minnesota Senatefrom the 36th districtIn officeJanuary 2, 1973 – November 5, 1974Preceded byGlenn D. McCartySucceeded byRobert J. Schmitz Personal detailsBornJames Frank LordNovember 26, 1948Chanhasse...

Historical form of Christian liturgy This article incorporates unedited text from the public-domain Catholic Encyclopedia. It may be out of date, or may reflect the point of view of the Catholic Church as of 1913. It should be edited to reflect broader and more recent perspectives. (August 2015) Part of a series on theCatholic ChurchSt. Peter's Basilica, Vatican City Overview Pope: Francis Hierarchy History (timeline) Theology Liturgy Sacraments Mary Background Jesus Crucifixion Resurrection ...

This article is about the 1940 Major League Baseball season only. For information on all of baseball, see 1940 in baseball. Sports season1940 MLB seasonLeagueMajor League BaseballSportBaseballDurationApril 27 – October 8, 1940Number of games154Number of teams16Regular seasonSeason MVPAL: Hank Greenberg (DET)NL: Frank McCormick (CIN)AL championsDetroit Tigers AL runners-upCleveland IndiansNL championsCincinnati Reds NL runners-upBrooklyn DodgersWorld SeriesChampionsCin...

Location of Mariposa County in California This is a list of the National Register of Historic Places listings in Mariposa County, California. This is intended to be a complete list of the properties and districts on the National Register of Historic Places in Mariposa County, California, United States. Latitude and longitude coordinates are provided for many National Register properties and districts; these locations may be seen together in a Google map.[1] There are 45 properties an...

В Википедии есть статьи о других людях с такой фамилией, см. Магдеев. Марат Фаикович Магдеев депутат Государственной думы Российской Федерации 29 декабря 2003 — 24 декабря 2007 Рождение 26 июля 1952(1952-07-26) (72 года)Бугульма, Татарская АССР, РСФСР, СССР Партия Либерально-демократи...

Dieser Artikel behandelt das Musikinstrument. Zu weiteren Bedeutungen siehe Klavier (Begriffsklärung) und Piano (Begriffsklärung). Klavier englisch: piano, italienisch: pianoforte Flügel und Pianino Klassifikation Chordophon Tasteninstrument Tonumfang Vorlage:Infobox Musikinstrument/Wartung/Parameter Klangbeispiel fehlt Verwandte Instrumente Celesta, Cembalo, Hackbrett Musiker Liste von Pianisten Kategorie:Pianist Klavier (von lateinisch clavis „Schlüssel“; mittellateinisch...

Pour les articles homonymes, voir Coupe de Russie. Coupe de Russie Généralités Sport Football Création 1992 Organisateur(s) RFS Périodicité Annuelle Lieu(x) Russie Statut des participants Professionnel Site web officiel www.rfs.ru Palmarès Tenant du titre Zénith Saint-Pétersbourg Plus titré(s) Lokomotiv Moscou (9) Pour la dernière compétition voir : Coupe de Russie de football 2023-2024 modifier La Coupe de Russie de football (en russe : Кубок России п�...

Pour les articles homonymes, voir Saphir (homonymie). SaphirCatégorie IV : oxydes et hydroxydes[1] Saphir à l'état brut. Général Numéro CAS 1317-82-4 Classe de Strunz 4.CB.05 4 OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates) 4.C Metal:Oxygen = 2:3, 3:5, and Similar 4.CB With medium-sized cations 4.CB.05 Tistarite Ti2O3Space Group R 3cPoint Group 3 2/m 4.C...

Grange-de-Vaivre La mairie de Grange-de-Vaivre. Administration Pays France Région Bourgogne-Franche-Comté Département Jura Arrondissement Dole Intercommunalité Communauté de communes du Val d'Amour Maire Mandat Claude Masuyer 2020-2026 Code postal 39600 Code commune 39259 Démographie Populationmunicipale 41 hab. (2021 ) Densité 24 hab./km2 Géographie Coordonnées 47° 00′ 13″ nord, 5° 50′ 30″ est Altitude Min. 245 mMax. 403 m...

Collective action by people in favor of a cause This article contains too many pictures for its overall length. Relevant discussion may be found on the talk page. Please improve this article by removing indiscriminate collections of images or adjusting images that are sandwiching text in accordance with the Manual of Style on use of images. (August 2024) (Learn how and when to remove this message) Monday demonstrations in East Germany (1989–1991) helped bring down the Berlin Wall. Part of t...

アメリカ文学(アメリカぶんがく、(英: American literature)とは、アメリカ合衆国の文学、及びそれらの作品や作家を研究する学問のこと。米国文学(べいこくぶんがく)、米文学(べいぶんがく)とも言う。また、イギリス文学と合わせて英米文学と呼ぶこともある。『English literature』の場合、英国や合衆国に限らず英語による各地域の文学を含むことがある。しか�...

South Korean manhwa A Returner's Magic Should Be SpecialCover of the first volume of the manhwa adaptation귀환자의 마법은 특별해야 합니다Gwihwanjaui Mabeob-eun Teugbyeolhaeya HabnidaGenreAdventure, fantasy[1]AuthorUsonanIllustratorWookjakgaPublisherD&C Media (South Korea)Kadokawa Shoten (Japan)English publisherNA: Yen PressWebtoonserviceKakaoPage (South Korea)Piccoma (Japan)Tappytoon (English)Original runMay 19, 2018 – presentVolumes6Anime television ...

Tomáš Eduard ŠilingerBorn(1866-12-16)16 December 1866Tučín, Austria-HungaryDied17 June 1913(1913-06-17) (aged 46)LuhačoviceOccupation(s)Politician and journalist Tomáš Eduard Šilinger (16 December 1866, Tučín – 17 June 1913, Luhačovice) was a Czech politician and journalist.[1][2] He was a member of the Augustinian Order of Brno. He was chief editor of the Czech Catholic newspaper Hlas in 1896. Life and work Šilinger was born on 16 December 1866 in Tučín ...